AstraZeneca Acquires New Lipid-Lowering Pre-Clinical Therapy
AstraZeneca has signed an exclusive licensing deal with CSPC Pharmaceutical Group Ltd (CSPC) to promote the development of an innovative early-stage small molecule Lipoprotein (a) (Lp(a)) disruptor. This asset has the potential to provide added advantages for patients dealing with dyslipidaemia. This agreement enhances the company's cardiovascular portfolio, aiming to tackle key risk factors contributing to chronic cardiovascular disease.
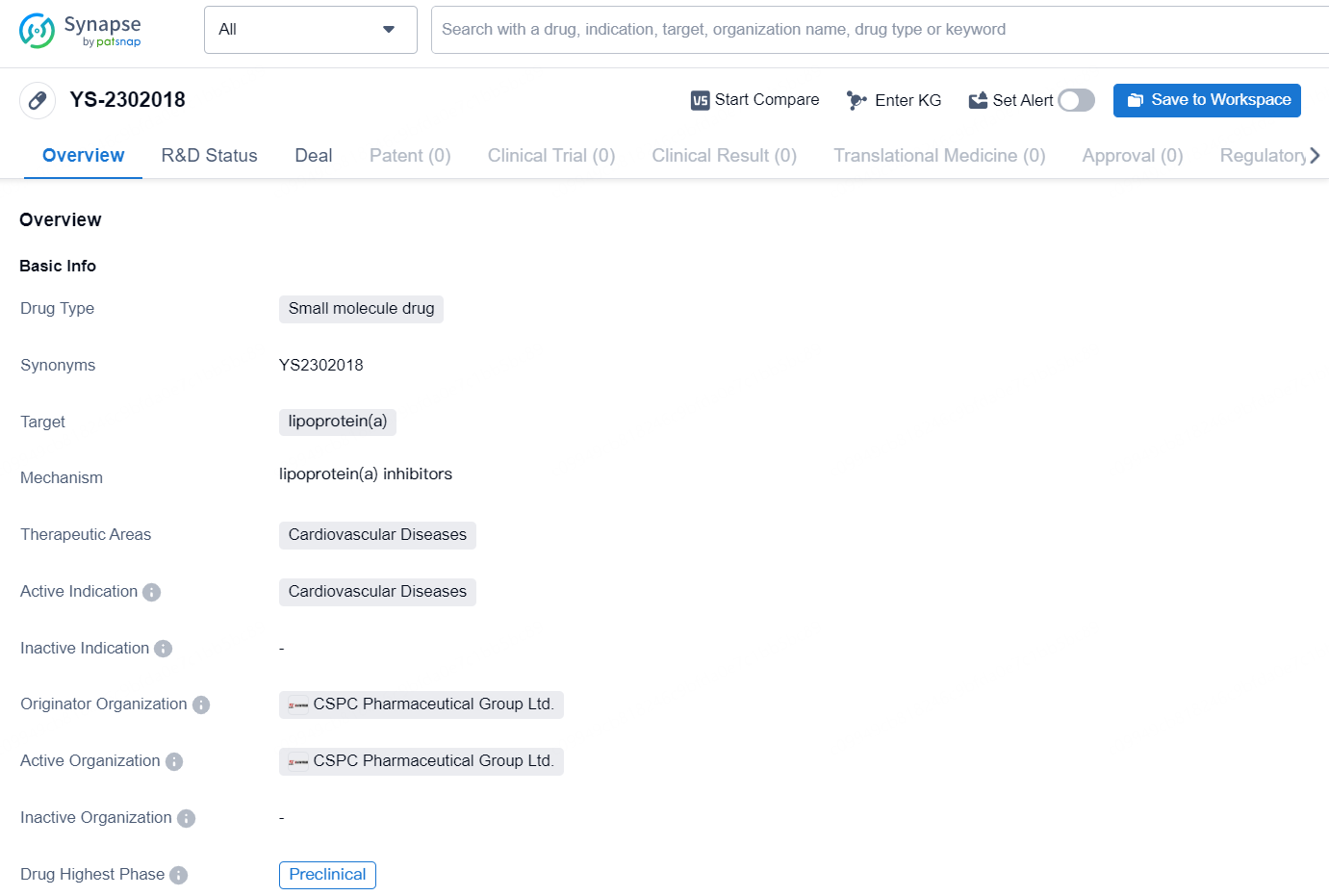
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Under this agreement, AstraZeneca will gain access to CSPC’s pre-clinical small molecule candidate, YS2302018. This compound is an oral Lp(a) disruptor, intended to be developed into an innovative lipid-lowering treatment with potential applications across various cardiovascular conditions, either as a standalone therapy or in combination, such as with the oral PCSK9 inhibitor, AZD0780.
CSPC discovered YS2302018, which has demonstrated effectiveness in hindering the formation of Lp(a). Lp(a) is a form of low-density lipoprotein (LDL) that is significant for cholesterol transport in the bloodstream. High levels of Lp(a), along with high LDL-cholesterol, are established risk factors for cardiovascular diseases, such as coronary artery disease and stroke.
Sharon Barr, Executive Vice President and Head of BioPharmaceuticals R&D at AstraZeneca, stated: "This asset represents a significant enhancement to our cardiovascular pipeline and may aid patients in better managing their dyslipidaemia and related cardiometabolic disorders. Considering the significant unmet need, with cardiovascular disease being a top global cause of death, developing innovative therapies that can effectively tackle known risk factors and improve patient care is crucial and central to our strategy."
Dongchen Cai, Chairman of CSPC Pharmaceutical Group Ltd, expressed, “Lipoprotein (a) is a crucial target for dyslipidemia and is involved in numerous cardiometabolic diseases. Through our agreement with AstraZeneca, and leveraging their expertise in clinical development and commercialisation at a global level, we are eager to expedite the advancement of YS2302018, a unique small molecule Lp(a) disruptor, to assist millions of patients worldwide affected by these conditions.”
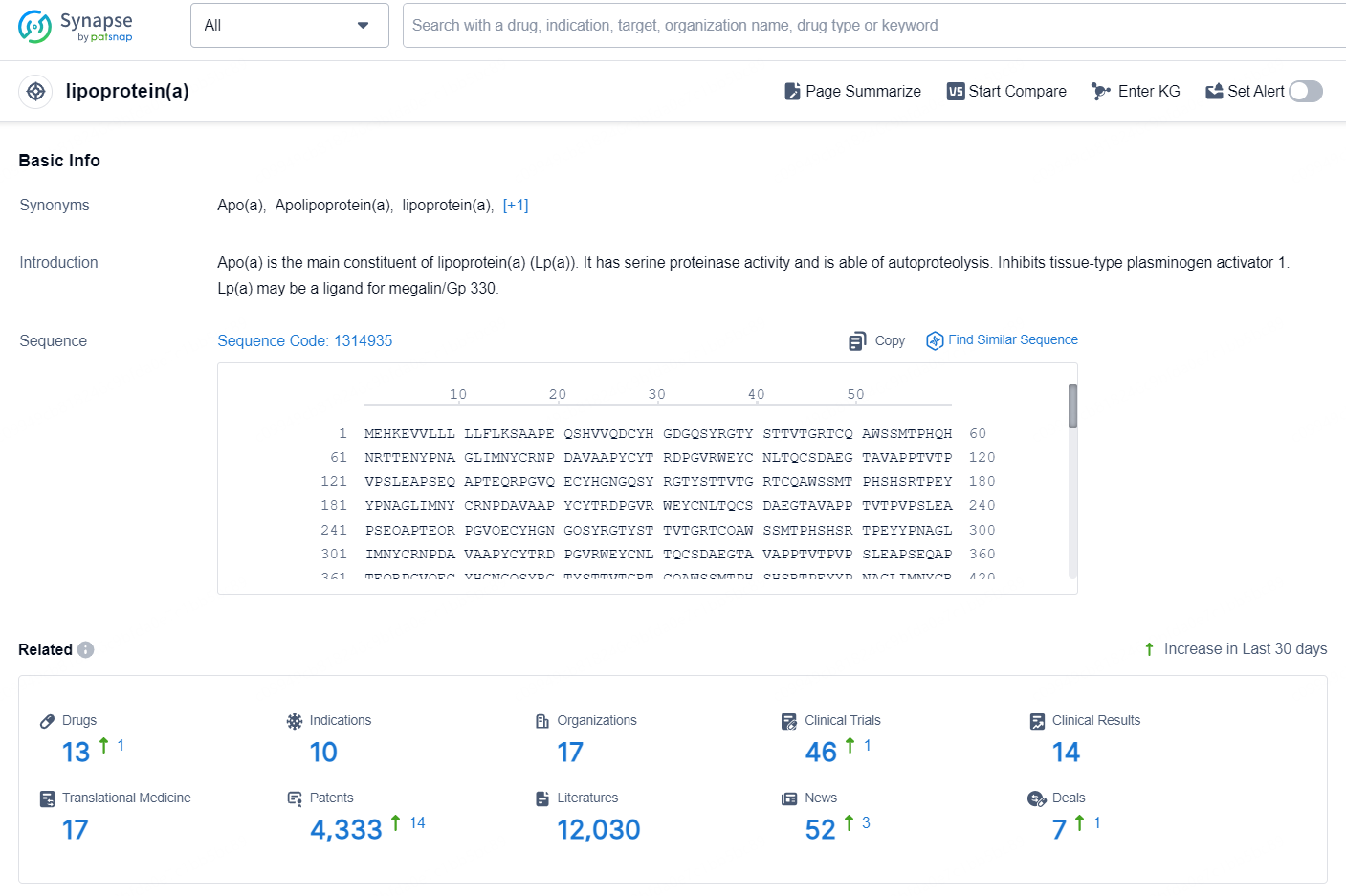
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 10, 2024, there are 13 investigational drugs for the lipoprotein(a) targets, including 10 indications, 17 R&D institutions involved, with related clinical trials reaching 46, and as many as 4333 patents.
The drug YS-2302018 is a small molecule drug that targets lipoprotein(a) and is intended for the treatment of cardiovascular diseases. It is currently in the preclinical phase of development, with the originator organization being CSPC Pharmaceutical Group Ltd. Lipoprotein(a) is a known risk factor for cardiovascular diseases, and the development of a small molecule drug targeting this protein represents a potential advancement in the treatment of such conditions. The therapeutic focus on cardiovascular diseases aligns with the global burden of these illnesses and the ongoing need for effective treatments..