First Dosing of Halia Therapeutics' Oral LRRK2 Inhibitor HT-4253 in Alzheimer's Study
Halia Therapeutics, a biopharmaceutical company focused on clinical-stage developments for new treatments targeting inflammatory and neurodegenerative conditions, has reported the initial administration of HT-4253, an LRRK2 inhibitor, to a healthy participant in their Phase 1 clinical study, which aims to assess its potential as a treatment for Alzheimer’s Disease.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Dr. David Bearss, CEO of Halia Therapeutics, announced that administering the initial dose to a participant in the Phase 1 clinical trial of HT-4253 signifies a pivotal advancement in addressing neurodegenerative diseases, particularly Alzheimer's. He emphasized that HT-4253 has the potential to be a revolutionary treatment for those with limited options to slow or halt Alzheimer's, given its focus on targeting the neuroinflammation associated with neurocognitive disorders.
The Phase 1 trial (ClinicalTrials.gov ID: NCT06537817) is a pioneering, randomized, double-blind, placebo-controlled study that investigates the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of HT-4253. The study uses a design incorporating both single ascending dose (SAD) and multiple ascending dose (MAD) approaches to assess HT-4253's ability to inhibit LRRK2, a vital enzyme involved in the disease.
Recent genetic research has identified certain Rab10 gene variants that offer protection to individuals with the APOE4 genotype, a recognized risk factor for Alzheimer's. These variants reduce neuroinflammation by hindering Rab10, a significant protein triggered by LRRK2. By blocking LRRK2, Halia's HT-4253 imitates these protective Rab10 variants, preventing Rab10 activation. This strategy holds promise for delaying or preventing Alzheimer's onset in individuals with the APOE4 genotype by mimicking the protective effects seen in naturally resilient individuals.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
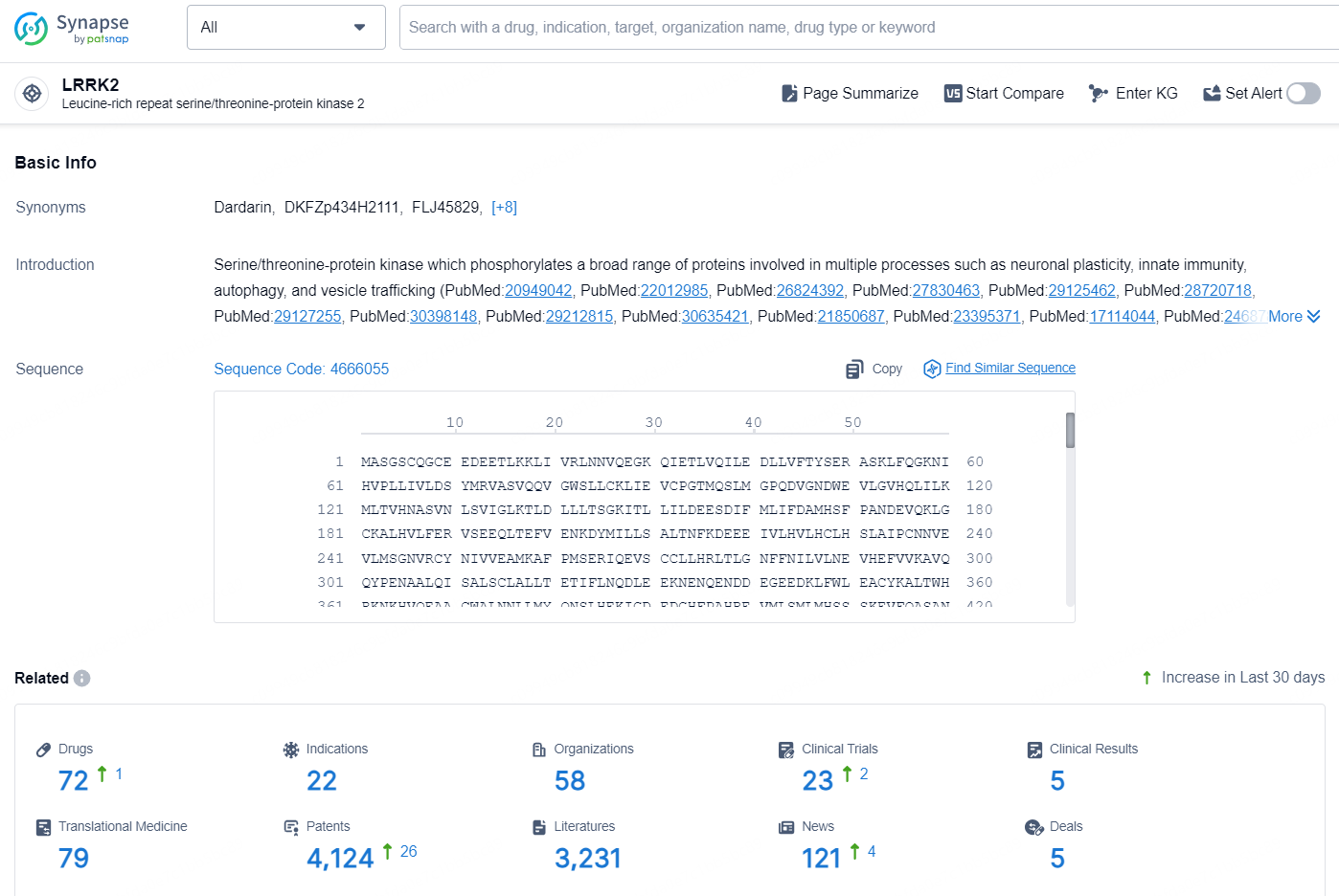
According to the data provided by the Synapse Database, As of October 10, 2024, there are 72 investigational drugs for the LRRK2 target, including 22 indications, 58 R&D institutions involved, with related clinical trials reaching 23, and as many as 4124 patents.
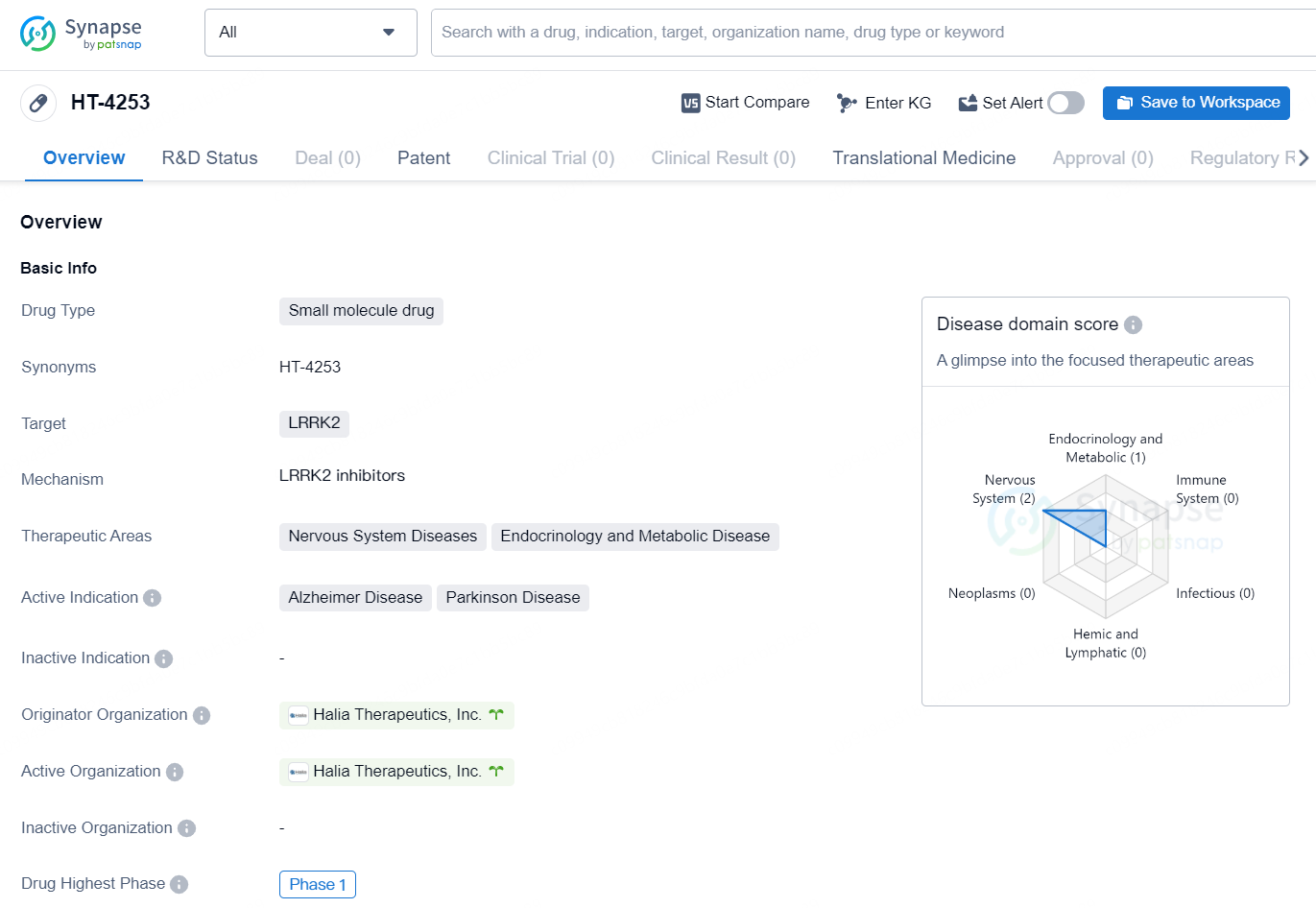
The small molecule drug, HT-4253, developed by Halia Therapeutics, Inc., targets LRRK2 and is being developed for the treatment of Nervous System Diseases and Endocrinology and Metabolic Diseases. The active indications for this drug include Alzheimer's Disease and Parkinson's Disease. HT-4253 has advanced to Phase 1 in its global development, indicating that it is currently undergoing preliminary testing in human subjects.