Intellia Launches Phase 3 HAELO Trial for CRISPR Treatment NTLA-2002 in Hereditary Angioedema
Intellia Therapeutics, Inc. (NASDAQ:NTLA), a prominent company in the clinical-stage of gene editing that is focused on transforming medicine through CRISPR-based treatments, has announced the launch of HAELO, an international and crucial Phase 3 trial for NTLA-2002 to address hereditary angioedema (HAE). NTLA-2002 is an entirely owned experimental in vivo CRISPR-based gene editing therapy, under development for administration as a single-dose treatment for this potentially dangerous condition. Intellia is actively screening patients after a successful end-of-Phase 2 discussion and submitting a modification to its Investigational New Drug Application to the U.S. Food and Drug Administration (FDA).
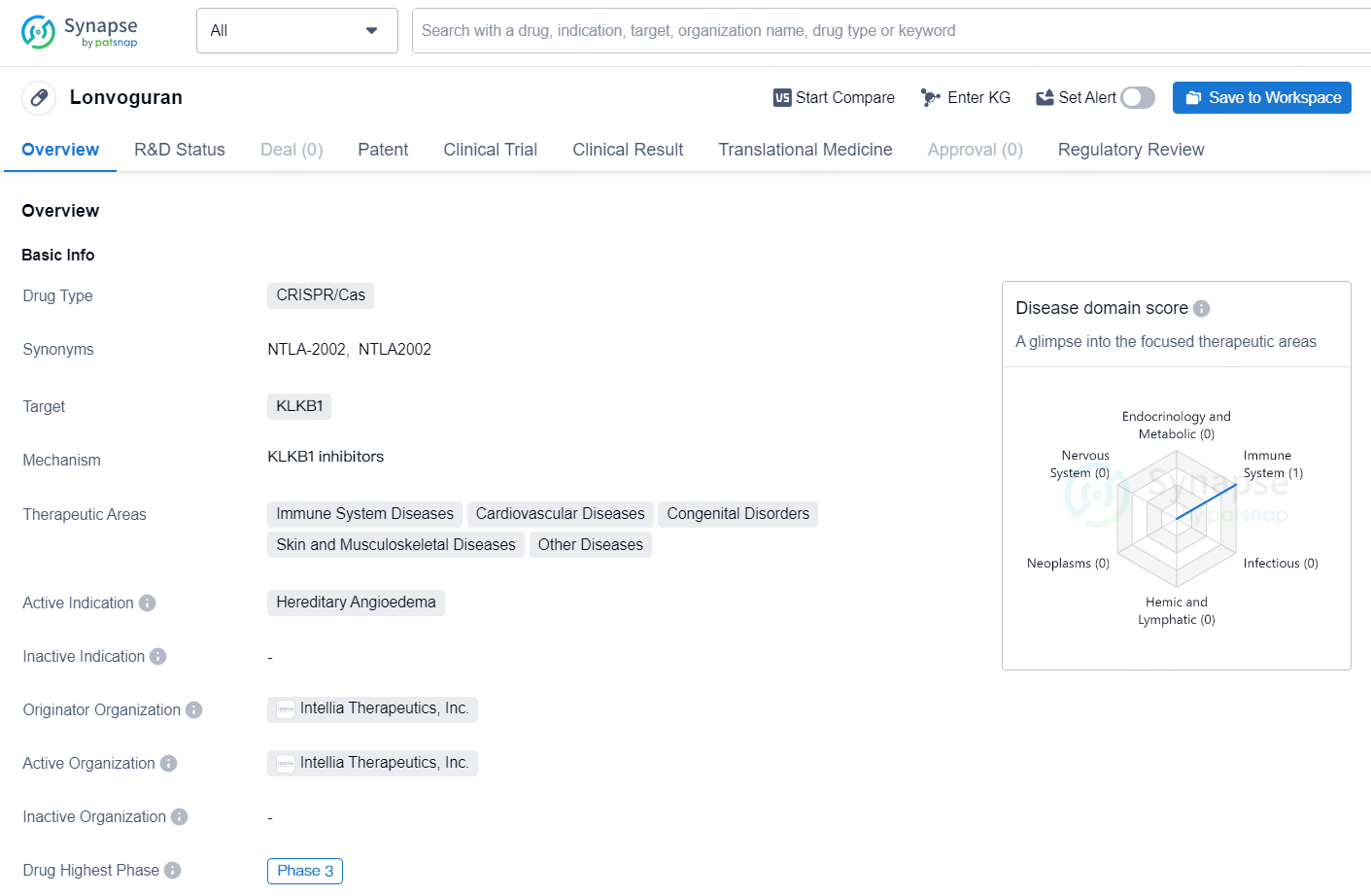
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The commencement of the HAELO Phase 3 clinical trial marks an important step forward for Intellia in the final phase of developing NTLA-2002 for individuals with hereditary angioedema," stated John Leonard, M.D., President and Chief Executive Officer of Intellia. "The ongoing Phase 1/2 study has provided highly encouraging data, suggesting that a single-dose therapy could result in a full response, eliminating attacks and the need for further treatment. We are working diligently to advance NTLA-2002 in order to meet the practical needs of those affected by this condition and ultimately anticipate it will offer considerable benefits to patients, healthcare providers, and insurers."
HAELO is an international, randomized, double-blind, placebo-controlled trial aimed at assessing the efficacy and safety of NTLA-2002 in 60 adults diagnosed with Type I or Type II HAE. Participants will be randomized in a 2:1 ratio to receive either a single 50 mg infusion of NTLA-2002 or a placebo. Those assigned to the placebo group will have the option to switch to NTLA-2002 treatment at week 28. The main endpoint is the change in HAE attack frequency from week 5 to week 28.
The Phase 3 trial is being launched by Intellia based on favorable safety and efficacy evidence from the ongoing Phase 1/2 study (NCT05120830) of NTLA-2002. Preliminary data from Phase 1 showed significant reductions in attack frequency, alongside consistent, profound, and lasting decreases in kallikrein levels. Positive topline results from Phase 2 of the study have previously been announced by Intellia. The company intends to present the comprehensive findings at the 2024 Annual Scientific Meeting of the American College of Allergy, Asthma & Immunology (ACAAI), scheduled for October 24 – 28 in Boston, Massachusetts.
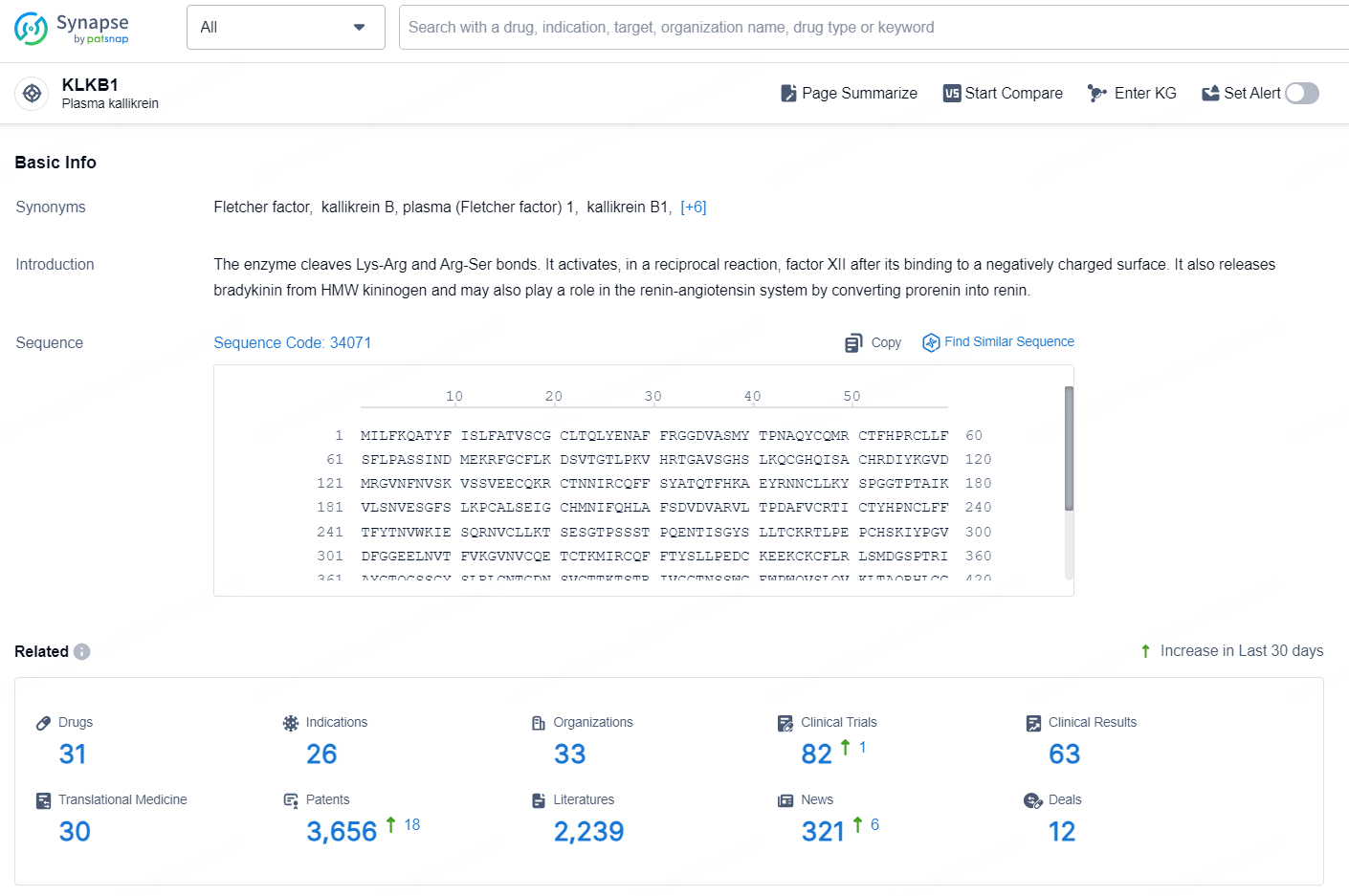
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 10, 2024, there are 31 investigational drug for the KLKB1 targets, including 26 indications, 33 R&D institutions involved, with related clinical trials reaching 82, and as many as 3656 patents.
Lonvoguran is a CRISPR/Cas drug developed by Intellia Therapeutics, Inc. The drug targets KLKB1 and is particularly focused on treating Hereditary Angioedema, a hereditary disorder that causes recurrent episodes of swelling in various parts of the body. The drug falls under the therapeutic areas of immune system diseases, cardiovascular diseases, congenital disorders, skin and musculoskeletal diseases, and other diseases.