Astria Therapeutics Reports Promising ALPHA-STAR Phase 1b/2 Results for HAE Using STAR-0215
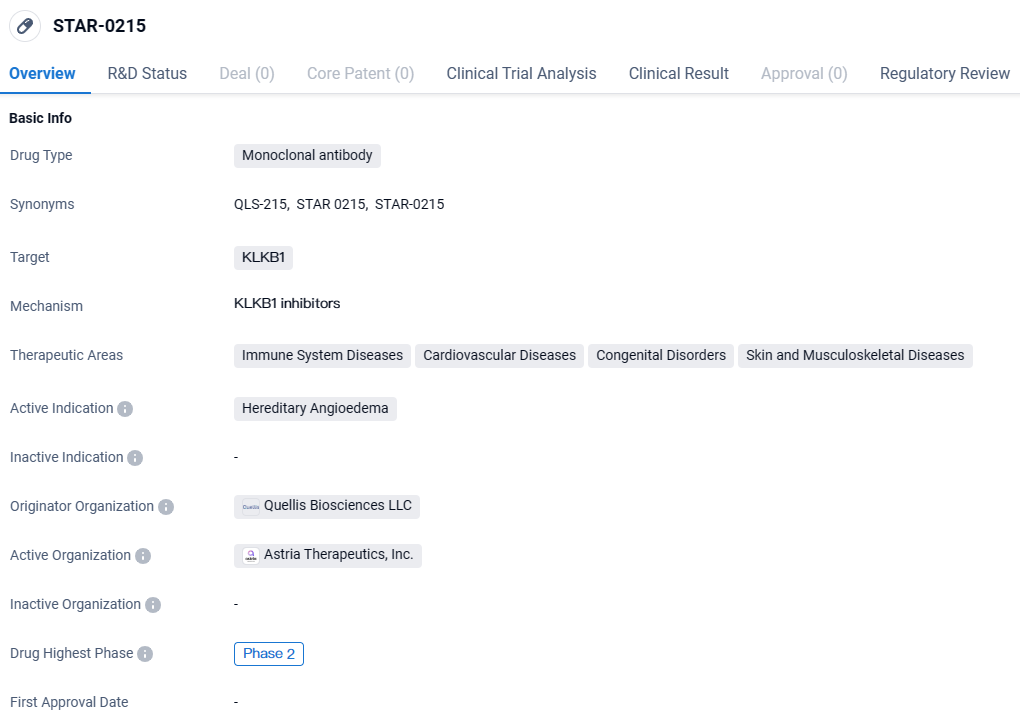
Astria Therapeutics, Inc., a company specializing in the creation of innovative treatments for conditions involving allergies and immune system disorders, has declared encouraging preliminary results from its early-stage ALPHA-STAR Phase 1b/2 study. This study investigates the efficacy of the investigational drug STAR-0215, a monoclonal antibody designed to target and inhibit plasma kallikrein activity, in individuals diagnosed with hereditary angioedema.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Preliminary findings reveal a promising safety and tolerability signature, with an average reduction in monthly attack frequency between 90% and 96% during a monitoring period of up to half a year. These findings provide evidence in favor of dosage plans administered tri-monthly (Q3M) and biannually (Q6M). With these encouraging outcomes, Astria is preparing to escalate STAR-0215 into the Phase 3 stage, expecting to commence trials in the first quarter of 2025, with preliminary findings projected by the close of 2026.
Christopher Morabito, M.D., the Chief Medical Officer at Astria Therapeutics, expressed great enthusiasm about the early data from the ALPHA-STAR study and the potential for STAR-0215 to significantly ease the burden of disease and treatment for patients. "We are confident that our results support administering STAR-0215 over intervals of three and six months, and we are eager to move forward to Phase 3 as swiftly as possible,” he added.
Marcus Maurer, M.D., Executive Director at the Institute of Allergology at Charite – Universitatsmedizin Berlin, remarked, “The initial findings from the ALPHA-STAR study signal a significant development in the treatment of HAE. STAR-0215 offers a promising approach for patients to handle their condition with a trusted mechanism, yet also provides a greatly enhanced dosing schedule and pain-free administration.”
Jill C. Milne, Ph.D., Chief Executive Officer, reported, “The ALPHA-STAR study's expedited enrollment, a testament to the high anticipation for STAR-0215, has allowed us to share these outcomes sooner than anticipated. Our goal is for STAR-0215 to become the preferred HAE therapy and I’m grateful to the HAE community and the Astria team for reaching this significant milestone today.”
The firm anticipates that its financial assets comprised of $246.5 million in cash, cash equivalents, and short-term investments as of December 31, 2023, plus $137.1 million from financing actions in early 2024, should be ample for funding operations well into mid-2027. This includes covering all activities related to the STAR-0215 initiative through the finalization of the anticipated Q3M Phase 3 pivotal trial, in addition to furthering the development of the STAR-0310 OX40 initiative up until the submission of an IND and the initial insights from an early Phase 1a trial.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
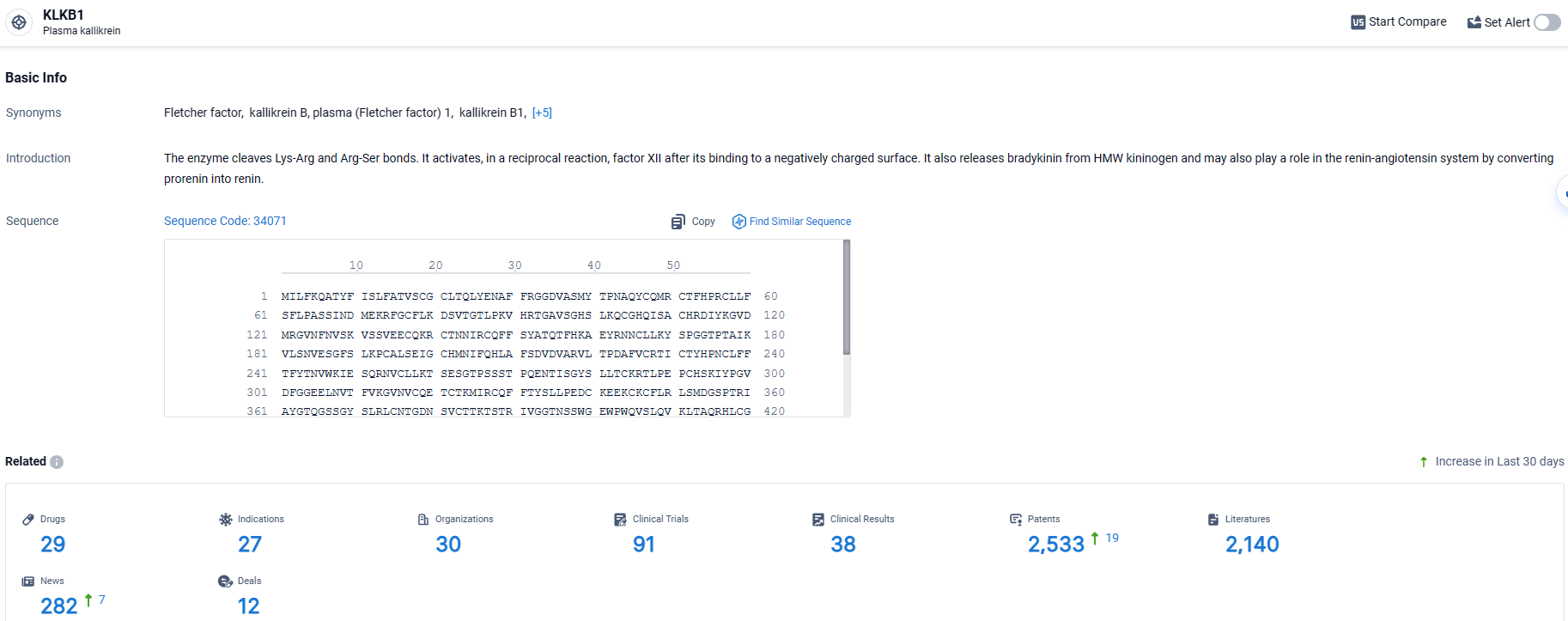
According to the data provided by the Synapse Database, As of March 26, 2024, there are 29 investigational drugs for the KLKB1 target, including 27 indications, 30 R&D institutions involved, with related clinical trials reaching 91, and as many as 2533 patents.
STAR-0215 targets KLKB1 and is being developed primarily for the treatment of Hereditary Angioedema. The drug also shows potential in addressing other therapeutic areas such as immune system diseases, cardiovascular diseases, congenital disorders, and skin and musculoskeletal diseases. With its Fast Track designation, STAR-0215 is expected to undergo expedited regulatory review. However, further clinical trials are needed to determine its safety and efficacy before potential approval and market availability.