Synapse Simplified: How to Find Carbamazepine Information
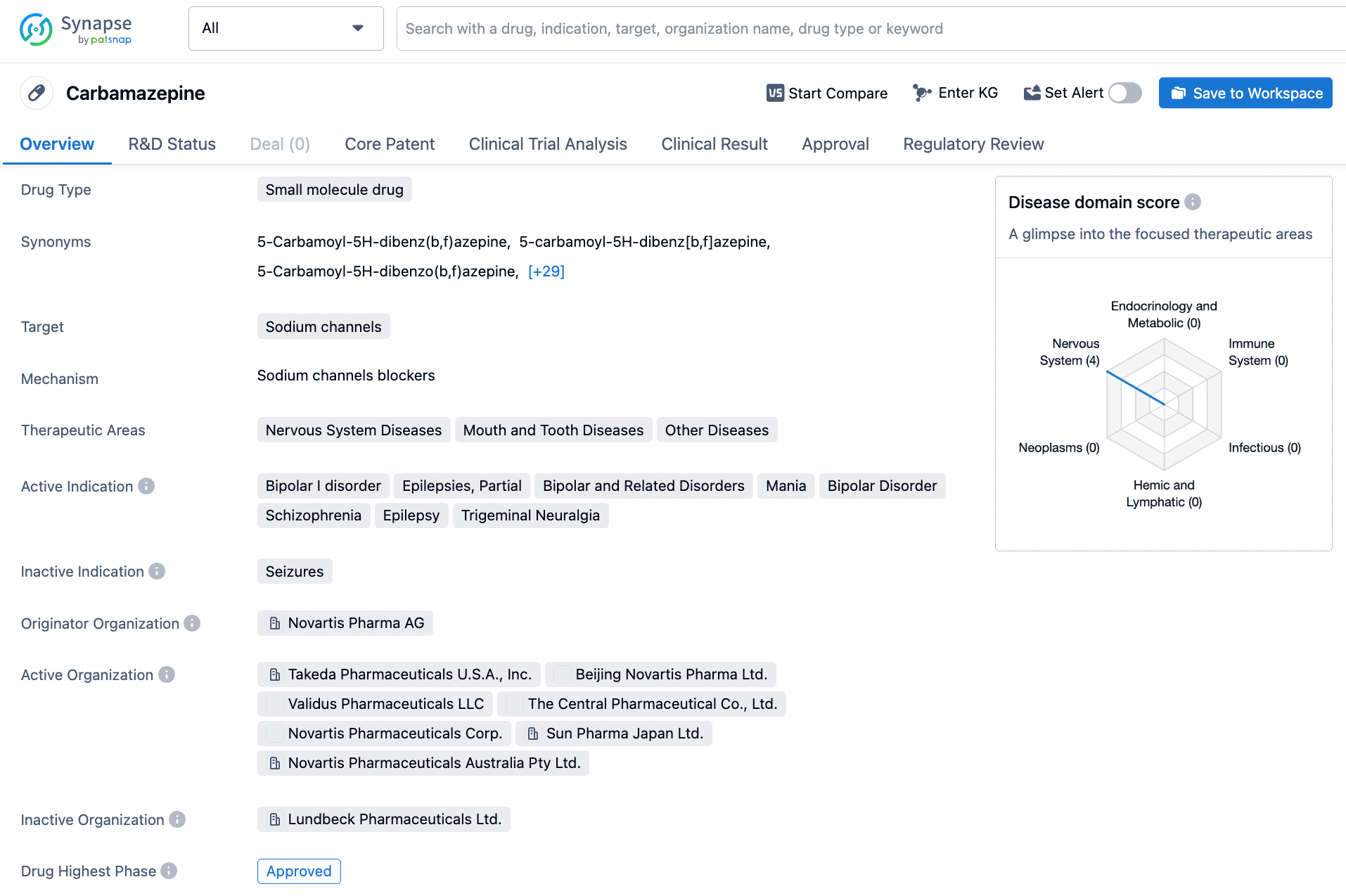
Carbamazepine, a minuscule molecule drug, exerts its therapeutic effect through the blocking of sodium channels in the human body. By virtue of this distinct mechanism of action, it is a valuable pharmacological agent in treating a plethora of disorders, such as bipolar I disorder, partial epilepsies, bipolar disorder, schizophrenia, epilepsy, and trigeminal neuralgia. It was initially synthesized and developed by the pharmaceutical company Novartis, and received its first regulatory approval in the month of March in the year 1965. The efficacy and safety of Carbamazepine in controlling seizures and mood swings associated with bipolar disorder is well-established. Furthermore, its analgesic properties in relieving neuropathic pain in trigeminal neuralgia have been extensively documented. Click on the image below to begin the exploration journey of Carbamazepine through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!