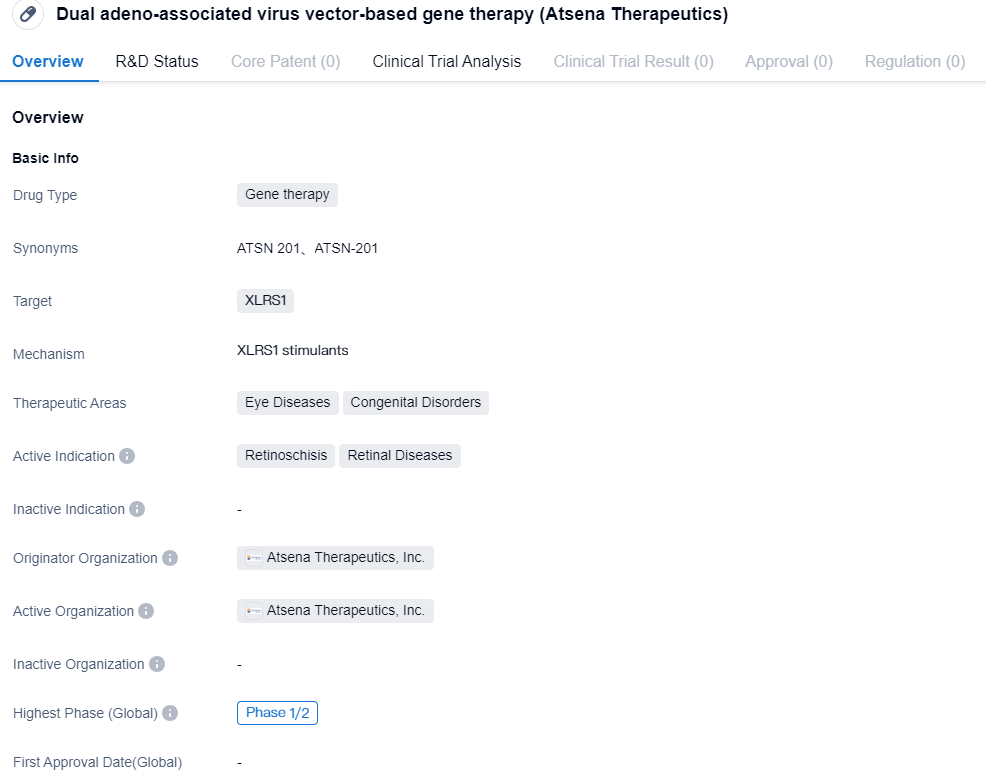
Atsena Therapeutics' ATSN-201 Phase I/II clinical trial has completed the first patient dosing
Atsena Therapeutics, a gene therapy company in the clinical-stage working towards harnessing the transformative power of genetical science to halt or reverse blindness, has revealed that the first patient has received a dose in its Phase I/II clinical trial, the LIGHTHOUSE study. This involves evaluating the subretinal injection of ATSN-201 for treating X-linked retinoschisis (XLRS). ATSN-201 takes advantage of AAV.SPR, the firm's cutting-edge spreading capsid, to elicit therapeutic levels of gene expression in the central retina's photoreceptors, sidestepping the surgical hazards associated with foveal detachment.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
“Dosing the first patient in the LIGHTHOUSE study marks a significant milestone for Atsena and the XLRS community,” commented Kenji Fujita, MD, Atsena Therapeutics' Chief Medical Officer. “We are thrilled to bring AAV.SPR to clinical use. It shows promise in revolutionizing XLRS treatment and other inherited retinal disorders. AAV.SPR expands laterally from the subretinal injection area, facilitating the secure delivery of RS1 to photoreceptors in the central retina/fovea. We eagerly anticipate propelling the LIGHTHOUSE study and the ongoing evolution of our innovative gene therapies aimed at preventing or reversing blindness.”
The LIGHTHOUSE investigation is a Phase I/II, open-label, dose-escalation, dose-expansion clinical trial that assesses the ATSN-201's safety and tolerability in male patients aged 6-64 with a clinical diagnosis of XLRS caused by destructive or possibly destructive mutations in RS1.
“In light of the absence of existing treatments for X-linked retinoschisis, this news is particularly encouraging for the inherited retinal disease community," Mark Pennesi, MD, PhD, Professor of Ophthalmology and Chief of the Paul H. Casey Ophthalmic Genetics Division Molecular and Medical Genetics, School of Medicine at Oregon Health & Science University noted.
About X-linked Retinoschisis (XLRS)
XLRS, a monogenic X-linked disorder caused by mutations in the RS1 gene, makes a protein mainly secreted by photoreceptors called retinoschisin. XLRS is typified by schisis, or an unusual separation of the retina's layers, leading to a vision impairment that glasses cannot correct and progressive vision loss. Males primarily suffer from XLRS, which is usually diagnosed in childhood. Roughly 30,000 males in the U.S. and EU have XLRS, for which no approved treatments currently exist.
About AAV.SPR
Atsena’s novel spreading capsid, AAV.SPR, stretches laterally beyond the subretinal injection area to enable safe and efficient conversion of the central retina. A non-human primate preclinical study showed that AAV.SPR promotes transgene expression well beyond the subretinal injection bleb boundaries.
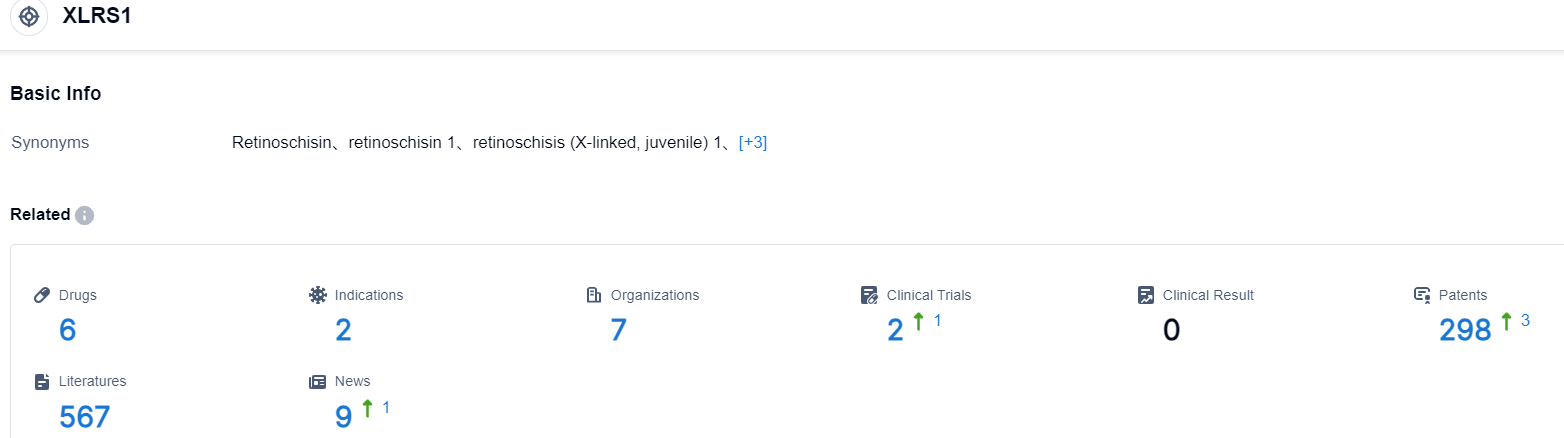
Click on the image below for direct access to the latest R&D progress on XLRS1 target drugs, indications, research institutions, clinical trials, and more. as of August 30, 2023, there are 6 drugs under research for the XLRS1 target, covering 2 types of indications, 7 research institutions involved, 2 related clinical trials, and as many as 298 patents. Atsena Therapeutics is a clinical-stage gene therapy company developing new treatments for inherited forms of blindness. The company has two clinical-stage programs and focuses on patients in a Phase I/II clinical trial known as the LIGHTHOUSE study. Ocular gene therapy pioneers Dr. Shannon Boye and Sanford Boye of the University of Florida founded Atsena, which is includes in North Carolina’s Research Triangle, an environment rich in gene therapy expertise.