Frovatriptan Succinate Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs
Frovatriptan Succinate's R&D Progress
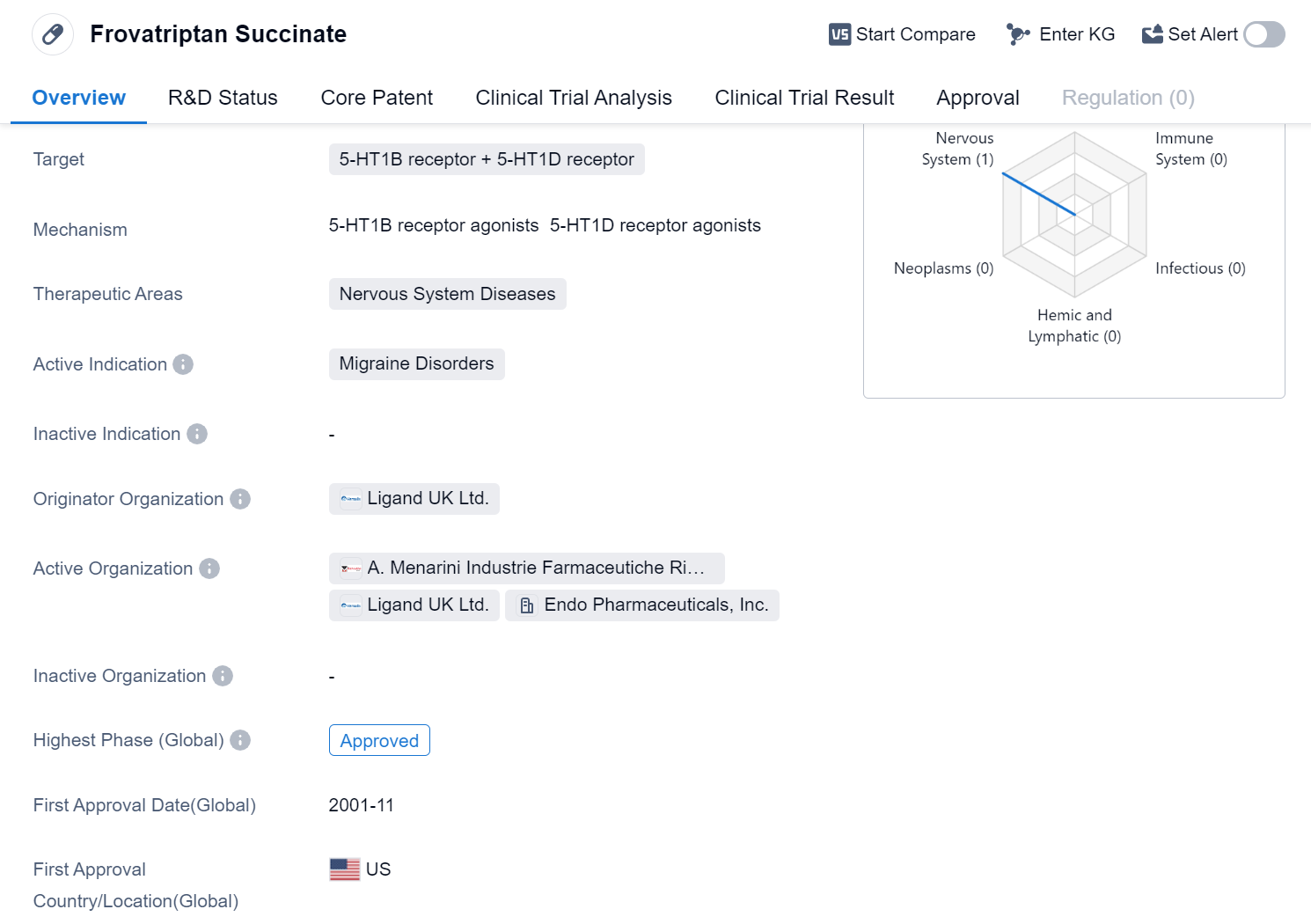
Frovatriptan Succinate is a small molecule drug that targets the 5-HT1B receptor and 5-HT1D receptor. It falls under the therapeutic area of Nervous System Diseases, specifically for the treatment of Migraine Disorders. The drug was first approved globally in November 2001 and the United States was the first country/location to grant approval.
Frovatriptan Succinate is developed by Ligand UK Ltd., an originator organization in the pharmaceutical industry. As a small molecule drug, it is designed to interact with specific receptors in the body to produce a therapeutic effect. In this case, the drug targets the 5-HT1B and 5-HT1D receptors, which are involved in the regulation of serotonin levels in the brain. By modulating these receptors, Frovatriptan Succinate aims to alleviate the symptoms associated with migraine disorders.
Migraine disorders are a type of neurological condition characterized by recurrent headaches, often accompanied by other symptoms such as nausea, sensitivity to light and sound, and visual disturbances. The approval of Frovatriptan Succinate provides healthcare professionals with an additional treatment option for patients suffering from migraines.
The highest R&D phase of Frovatriptan Succinate is approved. This milestone allows the drug to be marketed and prescribed to patients in need. The first approval of Frovatriptan Succinate in the United States suggests that it has met the regulatory requirements set by the U.S. Food and Drug Administration (FDA).
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Frovatriptan Succinate: 5-HT1B receptor agonists & 5-HT1D receptor agonists
5-HT1B receptor agonists and 5-HT1D receptor agonists are types of drugs that target specific serotonin receptors in the body.
From a biomedical perspective, the 5-HT1B and 5-HT1D receptors belong to the serotonin receptor family, which are G protein-coupled receptors found in the central nervous system. These receptors are involved in the regulation of various physiological processes, including mood, sleep, appetite, and pain sensation.
5-HT1B receptor agonists are drugs that bind to and activate the 5-HT1B receptors. By stimulating these receptors, they can have various effects such as vasoconstriction (narrowing of blood vessels), inhibition of neurotransmitter release, and reduction of pain transmission. These agonists are commonly used in the treatment of migraines and cluster headaches.
Similarly, 5-HT1D receptor agonists bind to and activate the 5-HT1D receptors. They also have vasoconstrictive effects and can inhibit the release of neurotransmitters. These drugs are primarily used for the treatment of migraines.
In summary, 5-HT1B receptor agonists and 5-HT1D receptor agonists are drugs that target specific serotonin receptors in the body. They are commonly used in the treatment of migraines and cluster headaches by exerting vasoconstrictive effects and inhibiting neurotransmitter release.
Drug Target R&D Trends for Frovatriptan Succinate
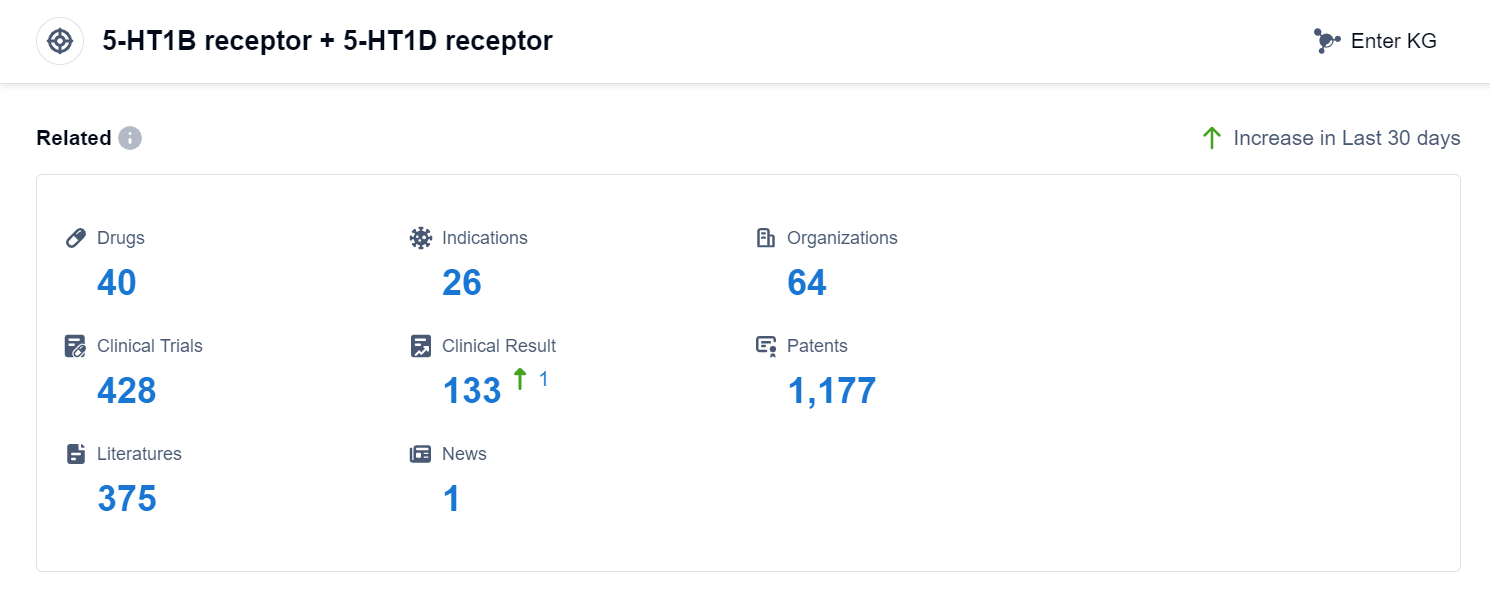
According to Patsnap Synapse, as of 4 Sep 2023, there are a total of 40 5-HT1B receptor + 5-HT1D receptor drugs worldwide, from 64 organizations, covering 26 indications, and conducting 428 clinical trials.
Based on the analysis of the provided data, the target 5-HT1B receptor + 5-HT1D receptor shows a promising competitive landscape in the pharmaceutical industry. Companies such as GSK Plc, SAWAI GROUP HOLDINGS Co., Ltd., and Eisai Co., Ltd. are leading in the development of drugs for this target. The most common approved indication for drugs targeting this target is Migraine Disorders, followed by Cluster Headache and Depressive Disorder, Major. Small molecule drugs are the most rapidly progressing drug type for this target. The United States, the European Union, China, and Japan are the countries/locations with the highest development progress for drugs targeting this target, with China showing significant progress. Overall, the target 5-HT1B receptor + 5-HT1D receptor presents opportunities for further development and innovation in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Frovatriptan Succinate is a small molecule drug developed by Ligand UK Ltd. It targets the 5-HT1B and 5-HT1D receptors and is approved for the treatment of migraine disorders. Its first approval was granted in the United States in November 2001, indicating its successful completion of the regulatory process. This drug provides healthcare professionals with an additional option to manage and alleviate the symptoms associated with migraines, benefiting patients suffering from this neurological condition.