The Advanced Journey of Glycemic Control Star - DPP-4 Inhibitors
The human intestinal tract can secrete a hormone called GLP-1, which is released after humans eat and promotes the secretion of insulin by pancreatic β cells, thus lowering blood sugar. Unfortunately, the half-life of GLP-1 is very short, and it can be easily hydrolyzed by an enzyme called DPP-4, thereby losing its activity.
DPP-4 inhibitors enhance the incretin effect by inhibiting DPP-4. The incretin effect refers to the phenomenon where the stimulation of insulin secretion by orally administered glucose is stronger than that by intravenous injection of glucose. This phenomenon exists because glucose entering the intestines orally can stimulate the intestinal mucosa to produce incretin.
Two types of intestinal incretins have been discovered so far: Glucagon-Like Peptide-1 (GLP-1) and Glucose-Dependent Insulinotropic Polypeptide (GIP), both of which enhance insulin secretion stimulated by glucose. GLP-1 and GIP are quickly degraded and inactivated in the body by DPP-4, in which endogenous DPP-4 plays a key role. DPP-4 inhibitors inhibit DPP-4, thereby increasing the level of endogenous active GLP-1 by 2 to 3 times, enhancing the sensitivity of β-cells and α-cells to glucose that increases insulin secretion stimulated by glucose and enhances the inhibitory effect of glucose on glucagon secretion, thereby improving hyperglycemia.
DPP-4 inhibitors can lower postprandial blood sugar, as well as fasting blood sugar. DPP-4 inhibitors can be used alone to treat type 2 diabetes, and can also be used in combination with other hypoglycemic drugs to treat type 2 diabetes. The most common hypoglycemic drugs combined with DPP-4 inhibitors are metformin, followed by sulfonylureas, thiazolidinediones, and insulin. DPP-4 inhibitors can also be used in combination with other hypoglycemic drugs for triple therapy. Common triple therapy regimens include DPP-4 inhibitors + metformin + sulfonylureas, DPP-4 inhibitors + metformin + thiazolidinediones, and DPP-4 inhibitors + metformin + insulin.
DPP-4 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 3 Sep 2023, there are a total of 143 DPP-4 drugs worldwide, from 153 organizations, covering 51 indications, and conducting 2227 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.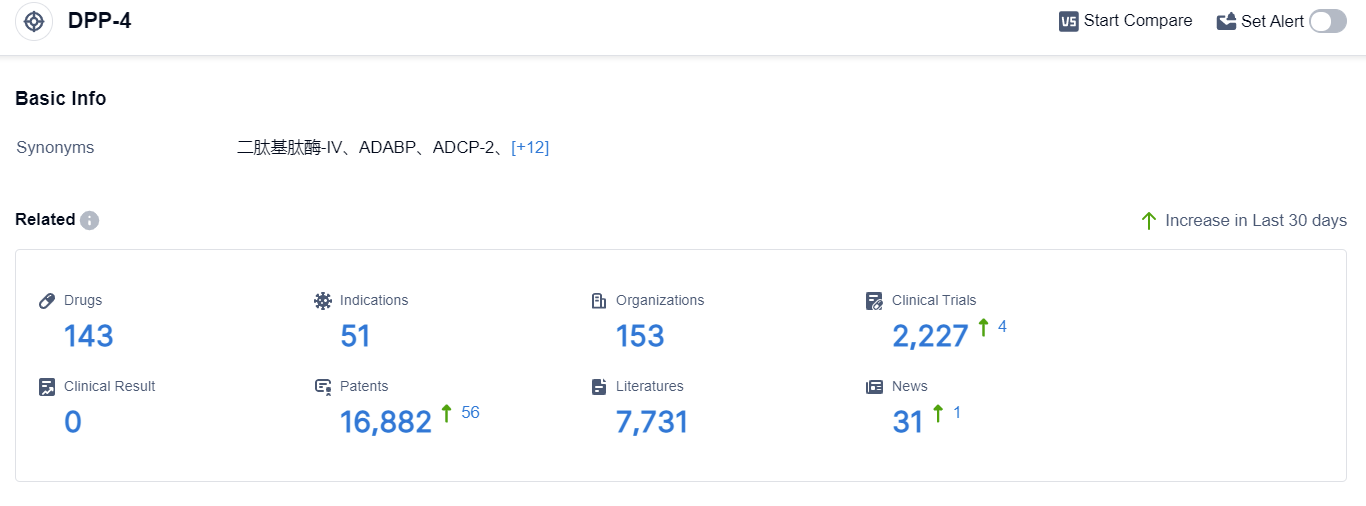
The analysis of the target DPP-4 in the pharmaceutical industry reveals a competitive landscape with several companies actively involved in the development of drugs. Merck & Co., Inc., Takeda Pharmaceutical Co., Ltd., C.H. Boehringer Sohn AG & Co. KG, AstraZeneca PLC, and LG Chem Ltd. are the companies growing fastest under the current target.
The most common indication for drugs targeting DPP-4 is Diabetes Mellitus, Type 2. Small molecule drugs are progressing most rapidly, with biosimilars indicating intense competition.
The European Union, Japan, the United States, South Korea, and China are the countries/locations developing fastest under the current target, with China showing significant progress.
Overall, the target DPP-4 presents a competitive landscape with a focus on addressing Diabetes Mellitus, Type 2, and the development of small molecule drugs.
The Outstanding Performer in DPP-4 Inhibitors - Linagliptin
As a critical limb of DPP-4 inhibitors, Linagliptin's hypoglycemic efficacy is definitive, accompanied with lesser hypoglycemic events and long-term cardio-renal safety confirmed by numerous studies. A meta-analysis incorporates three parallel, randomized, placebo-controlled Phase III clinical trials that lasted for 24 weeks. The integrated analysis indicates that, after 24 weeks, the baseline of HbA1c in Linagliptin patients dropped by 1.2%, 0.8% lower than the 0.4% drop in the placebo group (P<0.0001), conclusively proving Linagliptin's hypoglycemic effect.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In the CAROLINA study, the target for Linagliptin "high-quality standard" was set as HbA1c≤7.0%, no need for glucose-lowering rescue therapy, weight gain of ≤2%, and no occurrence of moderate/severe hypoglycemic events. The results showed that the proportion of Linagliptin "high-quality standard" was significantly higher than that of glimepiride (OR:1.68; 95.47%CI:1.43~1.96; P<0.0001), with more patients achieving high-quality standards with Linagliptin.
In addition, the dose of Linagliptin was 5mg/d, no dose adjustment was necessary. For the glimepiride group, the dose was 1-4mg/d, dose adjustments were needed, with an average dose during the study of (2.9±1.1)mg/d, 66% of the patients used the highest dose of 4mg.
Besides more patients achieving high-quality standards, the risk of hypoglycemic events in the Linagliptin group was also significantly lower than that of glimepiride. The CAROLINA study (with a median follow-up of more than 6 years) showed that compared with patients treated with glimepiride, the incidence of hypoglycemic events of varying degrees in patients treated with Linagliptin was significantly reduced (Linagliptin 10.6%, glimepiride 37.3%, HR:0.23; 95%CI:0.21~0.26, P<0.0001).
Furthermore, only 5% of Linagliptin is excreted through the kidneys, and only 10% is metabolized by the liver, with the metabolites having no pharmacological activity. Therefore, Linagliptin can be used in patients with mild, moderate, and severe hepatic/renal impairment without the need for dose adjustment.