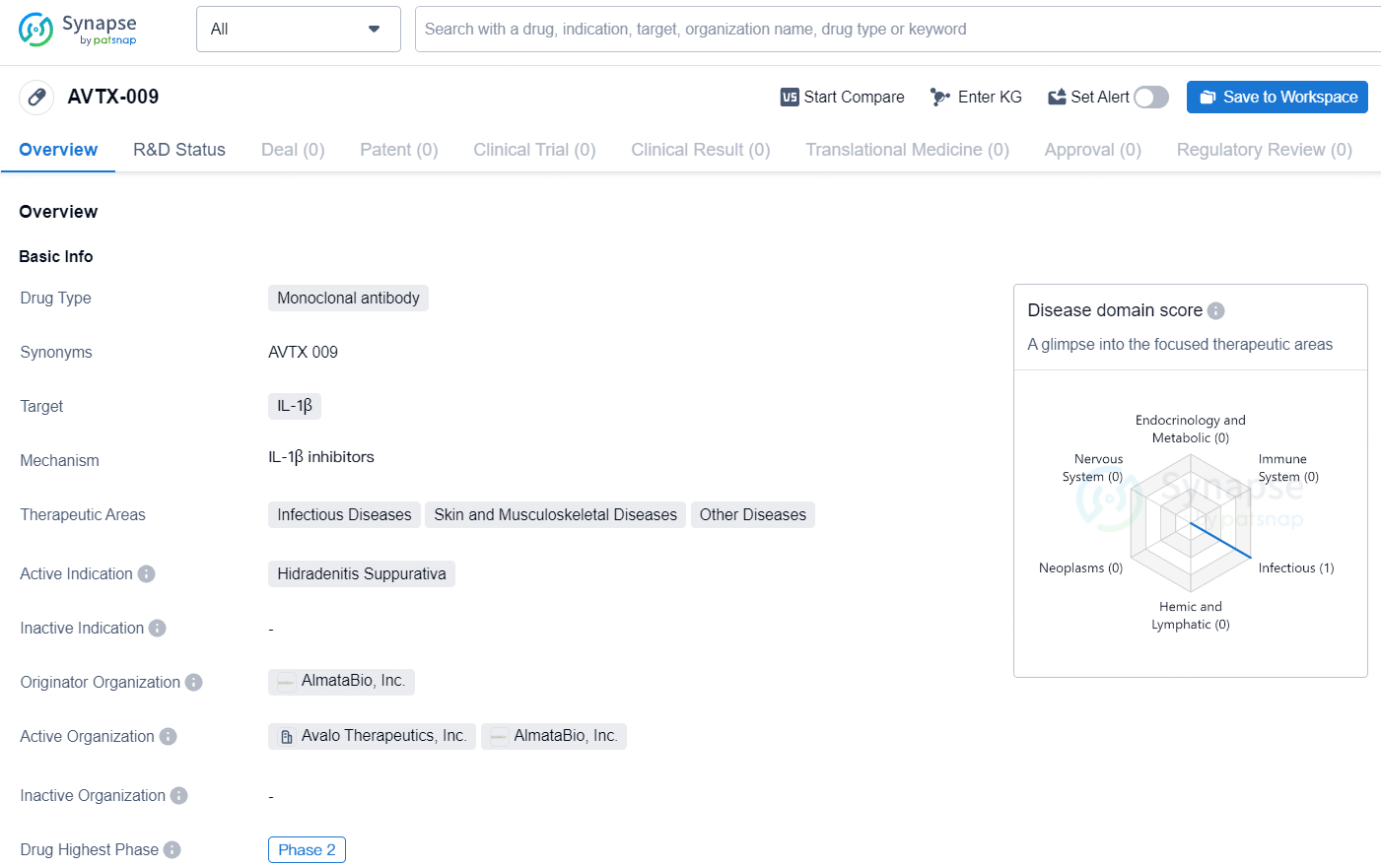
Avalo Therapeutics Announces IND Activation for AVTX-009, Targeting Hidradenitis Suppurativa Treatment
Avalo Therapeutics, Inc. has declared that the Investigational New Drug application for AVTX-009, an anti-IL-1β monoclonal antibody intended for the treatment of hidradenitis suppurativa, is now active. This approval allows the company to initiate its Phase 2 clinical trial in HS patients. Avalo anticipates enrolling the first patient in its Phase 2 LOTUS Trial within the year.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"This active IND marks a significant advancement towards initiating the LOTUS trial for patients suffering from hidradenitis suppurativa. I commend the Avalo team for accomplishing this within a little over three months after acquiring the product candidate in late March 2024," stated Dr. Garry Neil, CEO and Board Chairman. "We are optimistic that AVTX-009 could emerge as a leading option in its class and indication, thanks to its target, half-life, and potency, which might enable robust efficacy and convenient administration. We eagerly anticipate the launch of the LOTUS Trial."
The LOTUS Trial is designed as a randomized, double-blind, placebo-controlled, parallel-group Phase 2 study, aiming to evaluate the effectiveness and safety of AVTX-009 in approximately 180 adults with moderate to severe HS. The main efficacy measure is the percentage of participants achieving Hidradenitis Suppurativa Clinical Response by Week 16. Participants will be assigned randomly to receive one of two doses of AVTX-009 or a placebo.
AVTX-009 is a humanized monoclonal antibody (IgG4) that targets interleukin-1β (IL-1β) with high affinity, neutralizing its activity. IL-1β plays a crucial role in the inflammation process. Excessive production or misregulation of IL-1β is linked to several autoimmune and inflammatory conditions.
IL-1β is a recognized and important target for therapeutic approaches. There is substantial evidence indicating that IL-1β inhibition could be beneficial for treating HS as well as various inflammatory conditions in dermatology, gastroenterology, and rheumatology.
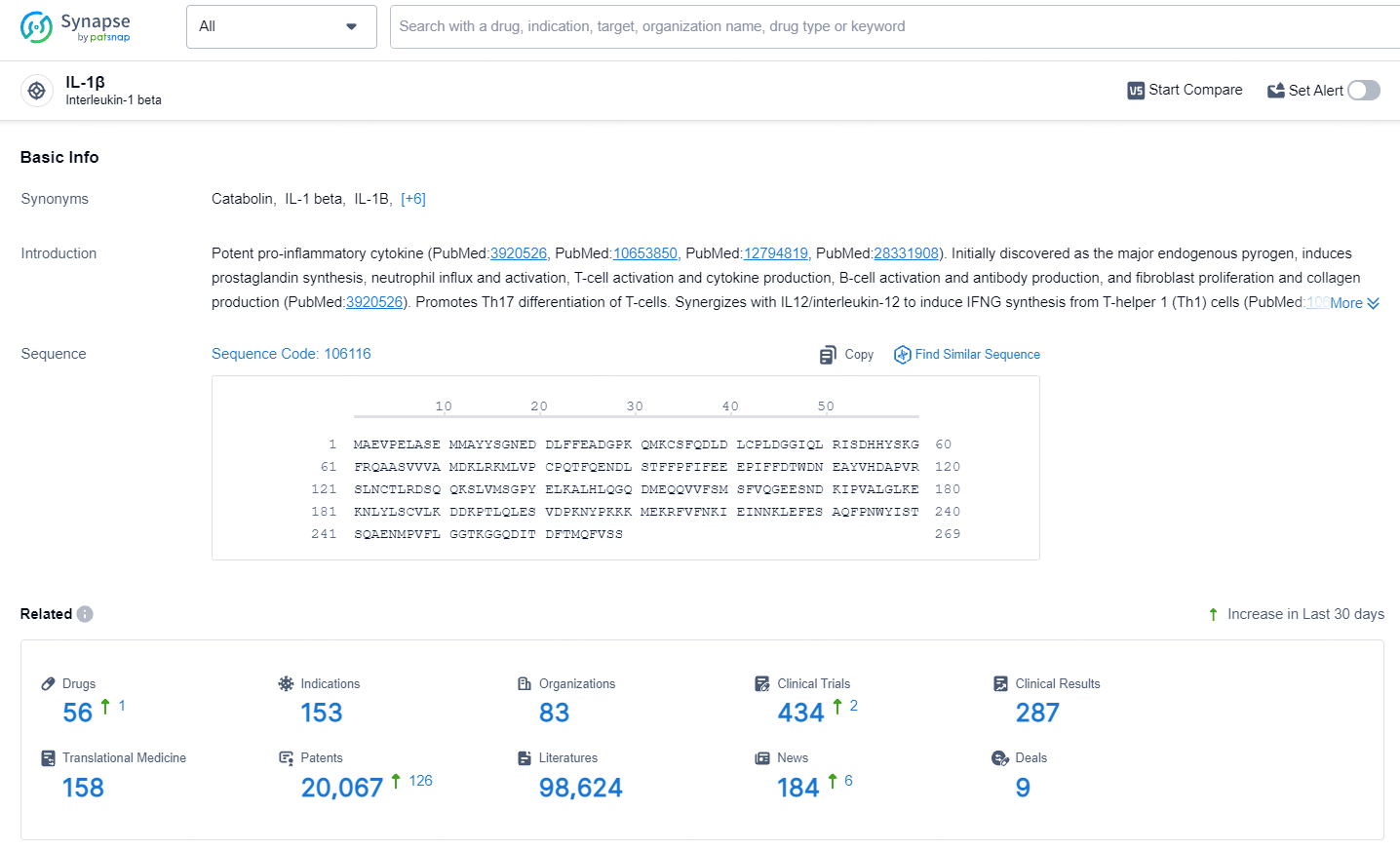
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of July 15, 2024, there are 56 investigational drugs for the IL-1β target, including 153 indications, 83 R&D institutions involved, with related clinical trials reaching 434, and as many as 20067 patents.
AVTX-009 target IL-1β with a monoclonal antibody highlights the potential of this drug to modulate the immune response and potentially address the underlying mechanisms of various diseases. AVTX-009 holds promise as a potential therapeutic option for patients suffering from Hidradenitis Suppurativa and other related conditions. Moving forward, further clinical trials and research are necessary to fully evaluate the safety and efficacy of AVTX-009 in its targeted therapeutic areas.