Prelude Therapeutics and Merck to Test PRT3789 with KEYTRUDA® in SMARCA4-Mutated Cancers
Prelude Therapeutics Incorporated, a precision oncology company currently in the clinical stage, announced its new clinical trial collaboration and supply deal with Merck. As stipulated in the agreement, the Phase 2 clinical trial will assess the efficacy of PRT3789—a novel, highly selective SMARCA2 degrader—in conjunction with Merck's anti-PD-1 therapy, KEYTRUDA (pembrolizumab), for patients suffering from cancers with SMARCA4 mutations.
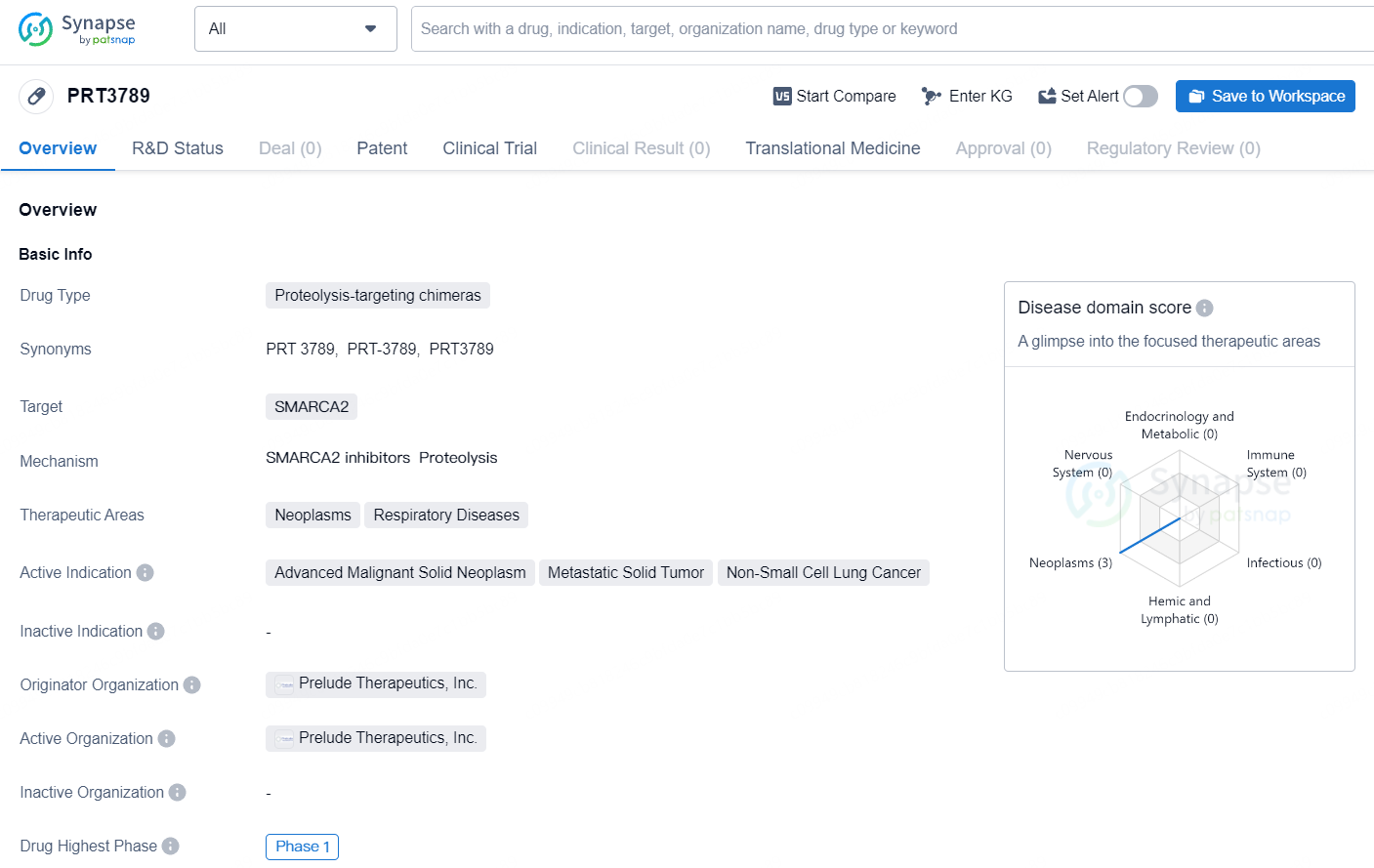
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“We are thrilled about the chance to partner with Merck for this study that merges our innovative, highly selective SMARCA2 degrader with KEYTRUDA,” said Jane Huang, M.D., President and Chief Medical Officer of Prelude. “This collaboration, which leverages potentially complementary mechanisms, could significantly improve clinical outcomes for patients with SMARCA4 mutations, who currently face limited treatment options.”
PRT3789 is a strong and highly selective, first-of-its-kind SMARCA2 degrader, now in Phase 1 clinical trials for patients with SMARCA4 mutations identified via biomarkers. The enrollment process is progressing well, and the Company anticipates concluding monotherapy dose escalation by mid-2024, thus establishing the recommended Phase 2 dose.
Additionally, enrollment continues for back-fill cohorts that are enriched for NSCLC and SMARCA4 loss-of-function mutations. The primary goals of this initial Phase 1 study are to determine the safety and tolerability of PRT3789, both as a monotherapy and in combination with docetaxel, as well as to assess its activity, pharmacokinetics, and pharmacodynamics. This study will also identify the appropriate dosage and potential indications for advancing into a registrational clinical trial.
The mechanistic rationale and pre-clinical data underscoring the combination of SMARCA2 degrader and anti-PD-1 monoclonal antibody were previously presented at the 2023 AACR International Conference on Molecular Targets and Cancer Therapeutics. In pre-clinical models, combining SMARCA2 degrader with an anti-PD-1 mAb in SMARCA4-mutated cancers enhanced anti-tumor immunity and showed tumor regressions.
According to the Agreement, Merck will supply KEYTRUDA to Prelude, which will act as the sponsor for the Phase 2 clinical combination trial. Both Prelude and Merck will retain all commercial rights to their respective compounds, whether used as monotherapy or in combination therapies.
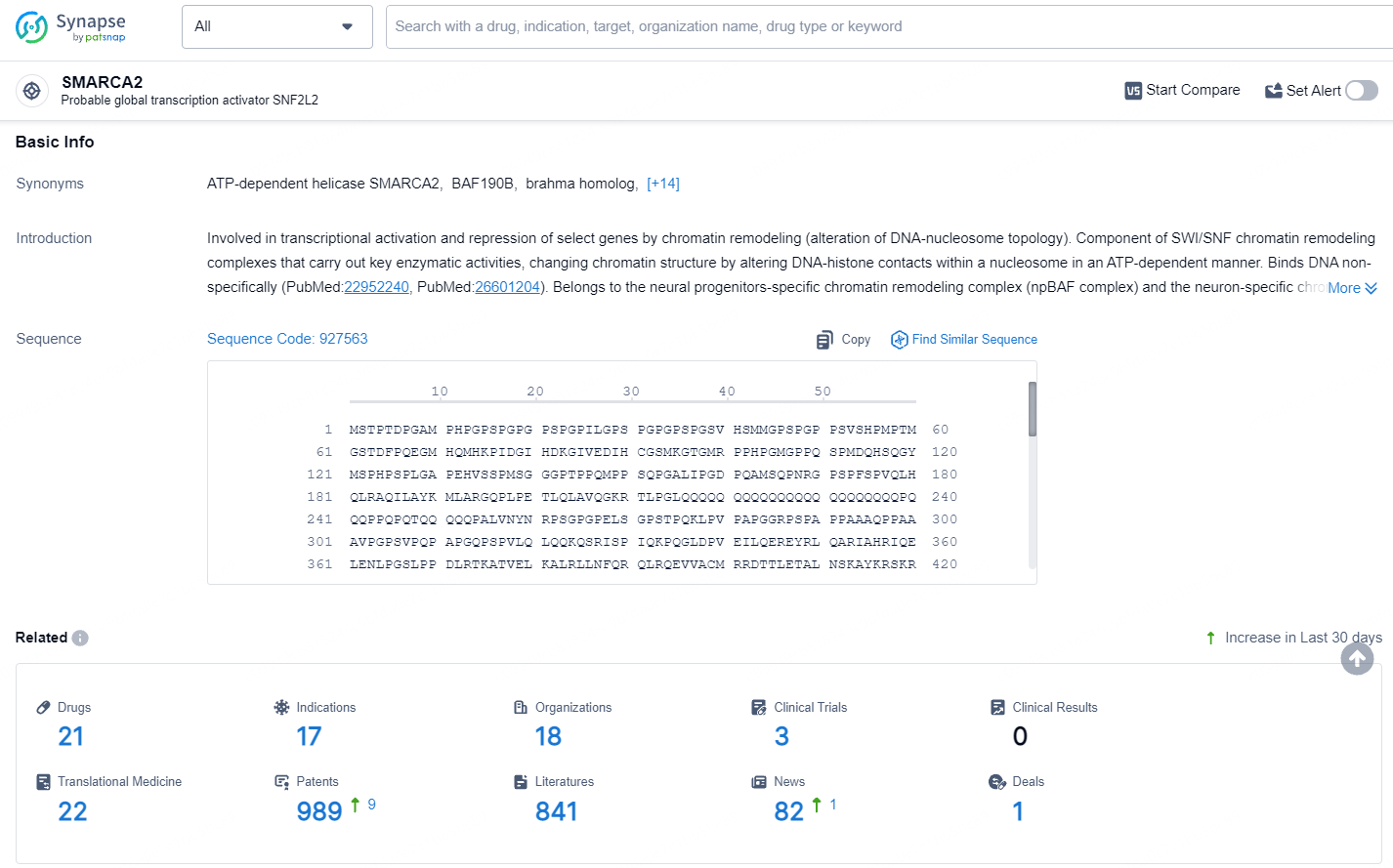
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 15, 2024, there are 21 investigational drugs for the SMARCA2 target, including 17 indications, 18 R&D institutions involved, with related clinical trials reaching 3, and as many as 989 patents.
PRT3789 is a proteolysis-targeting chimera drug developed by Prelude Therapeutics, Inc., with a focus on treating neoplasms and respiratory diseases, particularly advanced malignant solid neoplasm, metastatic solid tumor, and non-small cell lung cancer. The drug targets SMARCA2 and has reached Phase 1 of clinical trials, representing an early stage in its development and evaluation. As research and development in the field of biomedicine continue to advance, drugs like PRT3789 hold promise for addressing critical medical needs in the treatment of cancer and respiratory diseases.