uniQure Reports Positive Early Results in AMT-130 Phase I/II Trials for Huntington's Disease
uniQure N.V., a premier company in the gene therapy sector focused on developing breakthrough treatments for patients with critical medical conditions, has released new interim results. These results encompass up to 24 months of follow-up data from 29 patients who participated in the ongoing Phase I/II clinical studies of AMT-130 in the U.S. and Europe for treating Huntington’s disease.
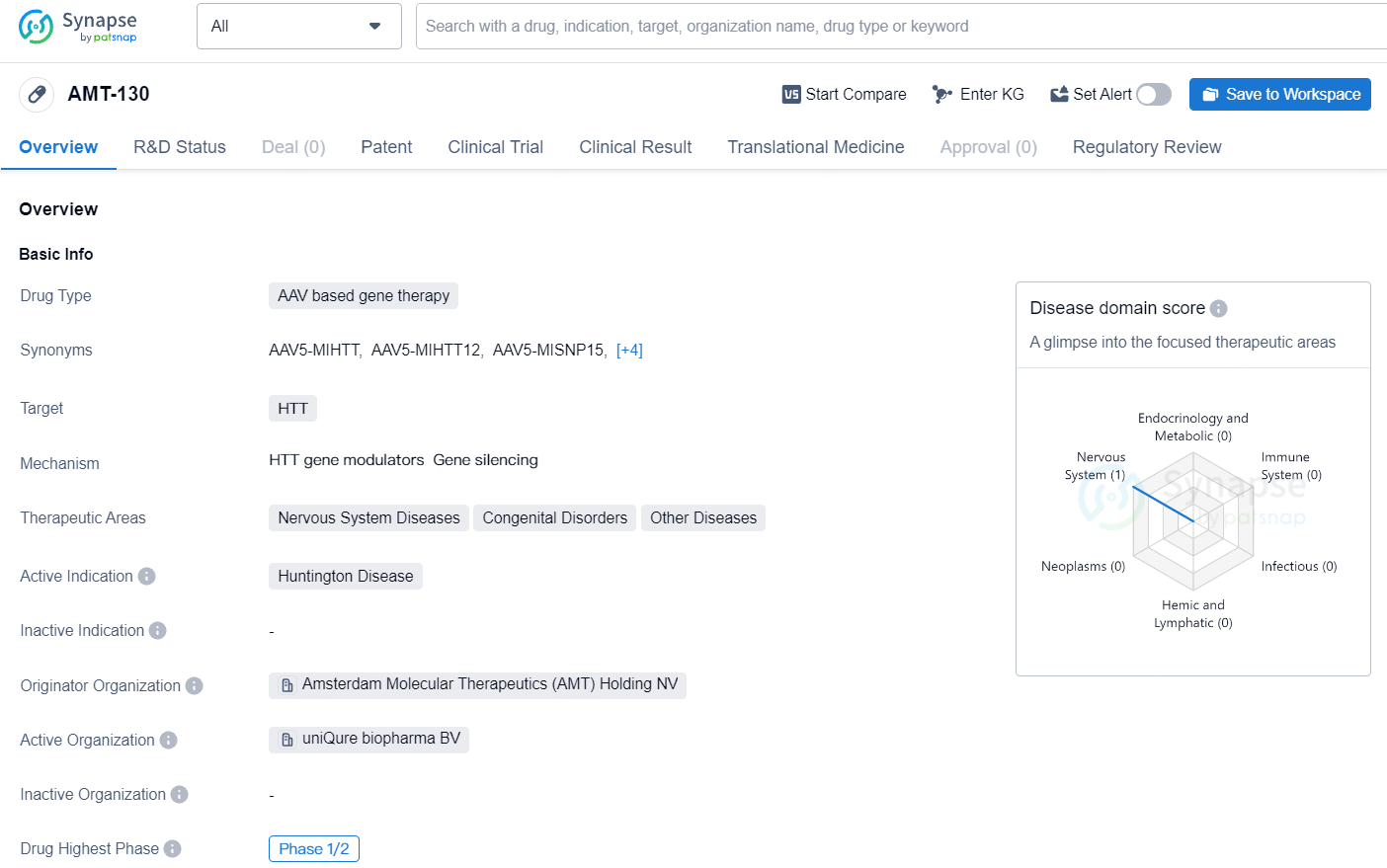
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"We are highly satisfied with the new data indicating a statistically significant, dose-dependent deceleration of Huntington’s disease progression and a reduction of NfL in the CSF after 24 months," said Walid Abi-Saab, M.D., the chief medical officer at uniQure.
"We believe this marks the first clinical trial of any experimental therapy for Huntington’s disease to suggest a potential long-term clinical benefit and a decrease in a critical neurodegeneration marker. Additionally, owing to the single administration of AMT-130, we are uniquely positioned to continue gathering long-term patient outcomes from the Phase I/II trials to bolster the emerging therapeutic effect. We anticipate an initial, multi-disciplinary RMAT meeting with the FDA later this year to discuss the potential for accelerated clinical development of AMT-130,” stated Walid Abi-Saab.
"These updated findings are thrilling and provide strong evidence of potential therapeutic advantage," remarked Victor Sung, M.D., professor of neurology at the University of Alabama at Birmingham, director of the UAB Huntington’s Disease Clinic, co-director of the UAB School of Medicine Neuroscience Module, and trustee on the National Board of the Huntington’s Disease Society of America.
"The maintenance of motor and cognitive function over two years, along with decreased NfL levels below baseline, contradicts the expected natural course of Huntington’s disease. cUHDRS, in particular, has been demonstrated to be a robust and sensitive measure of disease progression and enhances trial efficiency compared to individual assessments. These extended data give hopeful evidence of durable disease modification and provide much-needed optimism for a community urgently needing therapeutic options,” Victor Sung, M.D. added.
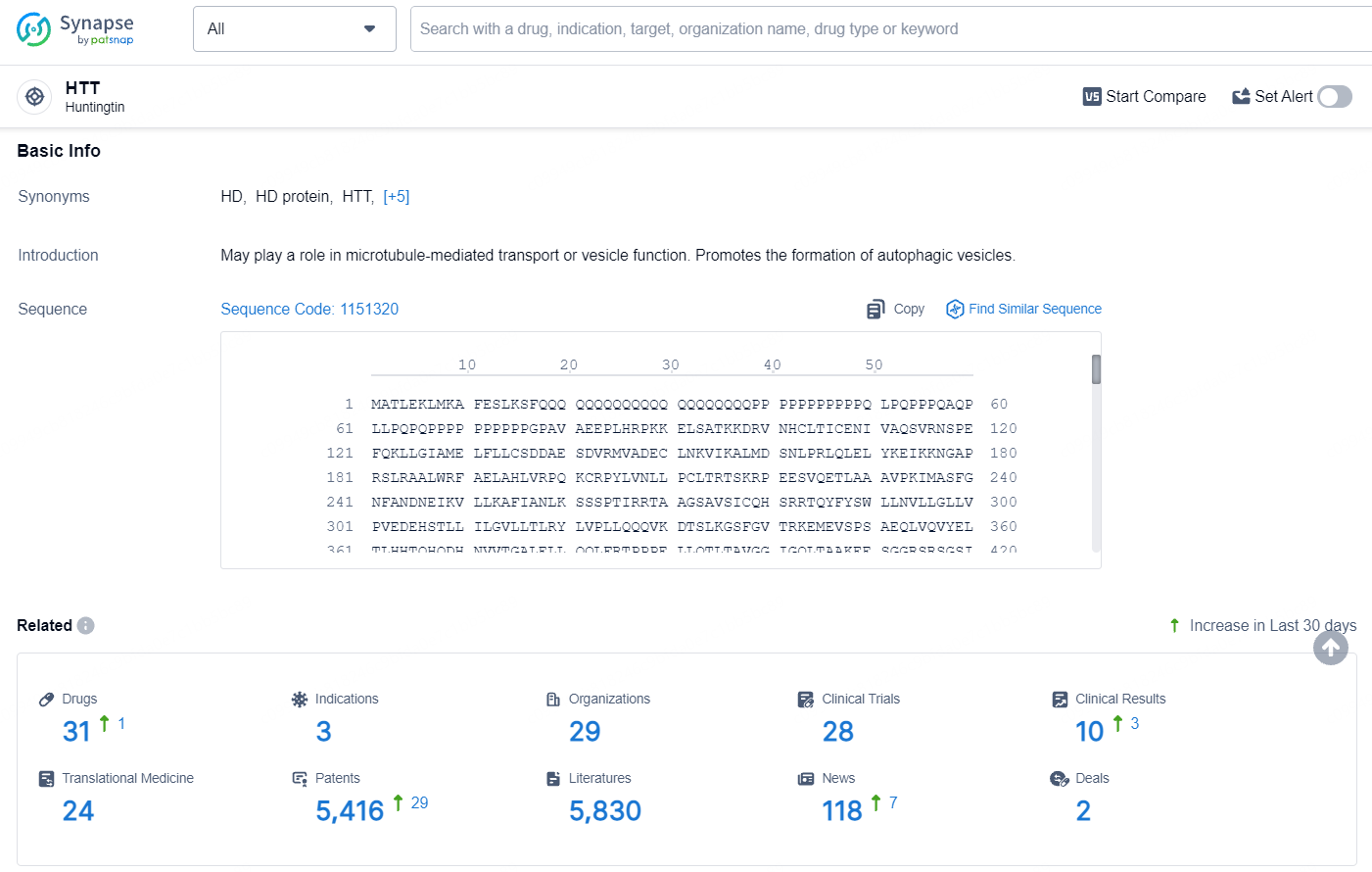
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 15, 2024, there are 31 investigational drugs for the HTT target, including 3 indications, 29 R&D institutions involved, with related clinical trials reaching 28, and as many as 5416 patents.
AMT-130 targets the HTT gene and primarily intended for the treatment of Huntington Disease. With its advancement to the Phase 1/2 stage of clinical development and regulatory designations as a Regenerative Medicine Advanced Therapy and Orphan Drug, AMT-130 holds promise as a potential therapeutic option for patients with Huntington Disease and related nervous system disorders.