Avenzo Therapeutics unveils promising CDK2 inhibitor AVZO-021, originally from Allorion Therapeutics
Allorion Therapeutics Inc., which operates in the biotech sector and is focused on advancing clinical trials for innovative cancer treatments, has disclosed the signing of a definitive licensing deal granting exclusive rights to Allorion Therapeutics Inc. This agreement is for the progression and market release of the drug candidate AVZO-021, previously known as ARTS-021.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
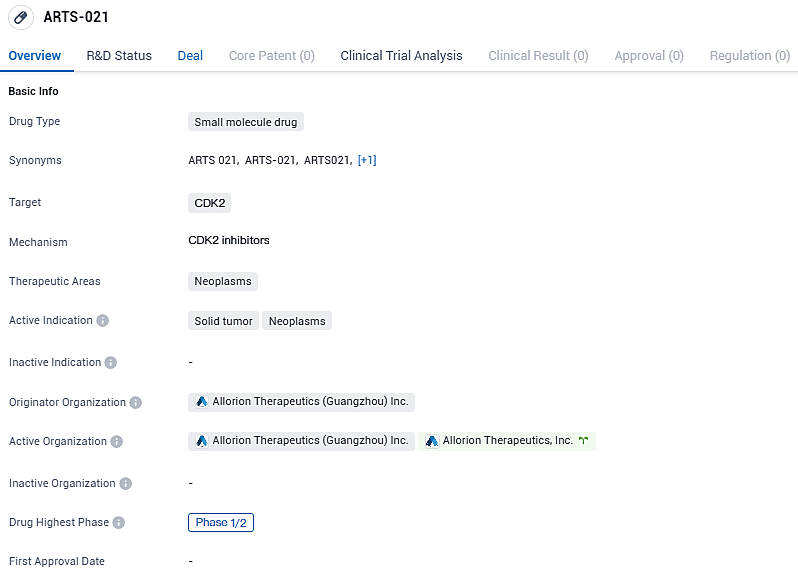
Globally, AVZO-021 is emerging as a lead contender among cyclin-dependent kinase 2 (CDK2) selective inhibitors. Under the recent deal, Avenzo secures an exclusive option to further a preclinical project, which is slated for Investigational New Drug (IND) application by the start of 2025.
CDK2 is becoming a coveted therapeutic target due to its function in circumventing resistance to FDA-sanctioned CDK4/6 treatments for hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2-) breast cancers and its oncogenic role in a range of cancers with cyclin E1 amplification. Avanzo is committed to applying its proprietary drug advancement capabilities to progress AVZO-021, which is under evaluation through a Phase 1 clinical study in various U.S. locations, to address HR+/HER2- metastatic breast cancer and additional serious solid malignancies.
Dr. Athena Countouriotis, co-founder, president, and CEO of Avenzo, commented, "This partnership marks a pivotal step in establishing our superior oncology portfolio. We aim to fill the gap in treatments available for patients suffering from HR+/HER2- metastatic breast cancer with AVZO-021, poised as a potential stand-alone therapy or in various combinations. The progression of the ongoing Phase 1 clinical trial and our collaboration with Allorion to propel this endeavor on an international scale is thrilling."
Through the licensing agreement’s stipulations, Allorion is set to receive an immediate $40 million payment and may qualify for more compensation tied to specific development, regulatory, and sales milestones and escalating royalties on Avenzo's net sales. The cumulative financial incentives for both agreements could exceed $1 billion.
Researchers found that in preclinical assessments, AVZO-021 has nanomolar efficacy in interactions with CDK2, and exhibits remarkable selectivity over CDK1, which is associated with adverse side effects. Additionally, AVZO-021 proved effective in vivo in tumor models when used both independently and in combination with CDK4/6 inhibitors. Allorion showcased selected findings at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics in October 2023 through poster sessions.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
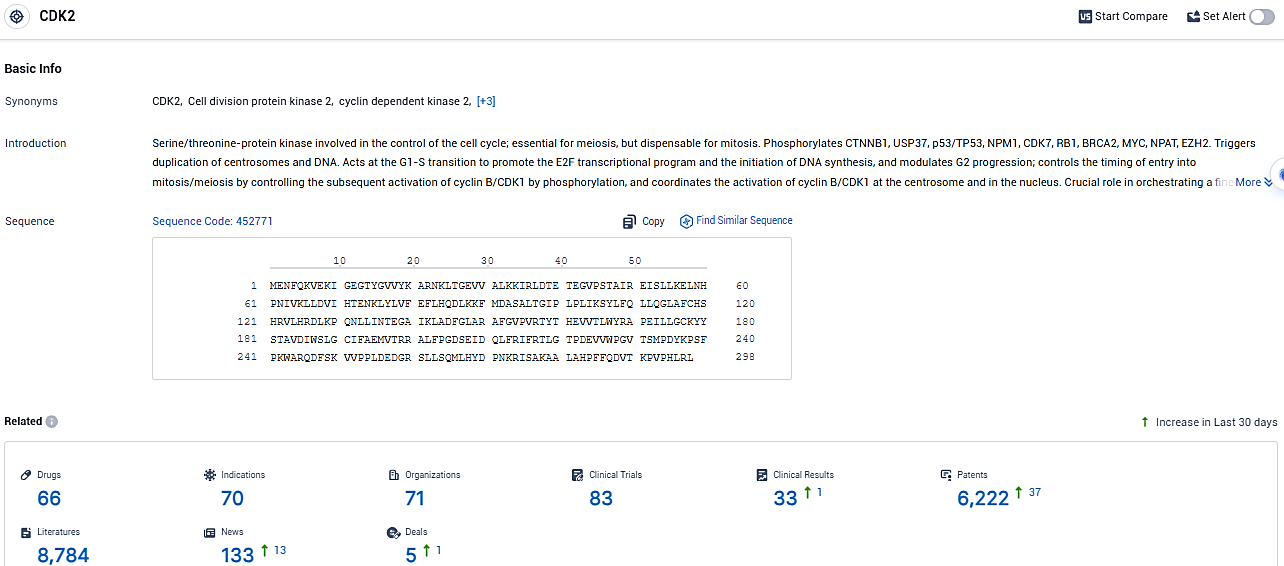
According to the data provided by the Synapse Database, As of January 10, 2024, there are 66 investigational drugs for the CDK2 target, including 70 indications, 71 R&D institutions involved, with related clinical trials reaching 83, and as many as 6222 patents.
Based on the available information, ARTS-021 shows promise as a potential treatment for neoplasms, particularly solid tumors. However, it is important to note that the drug is still in the early stages of development, and further clinical trials will be needed to determine its safety and efficacy.