Avidity Biosciences presents encouraging results for AOC 1001, showing progress and long-term safety in multiple areas for Type 1 Myotonic Dystrophy patients
Avidity Biosciences, Inc., a firm in the biopharmaceutical sector dedicated to providing a unique class of RNA therapeutics known as Antibody Oligonucleotide Conjugates, recently revealed promising AOC 1001 data. The data demonstrated enhancement in numerous further functional endpoints and showed positive extended safety and tolerability for individuals affected by myotonic dystrophy type 1.
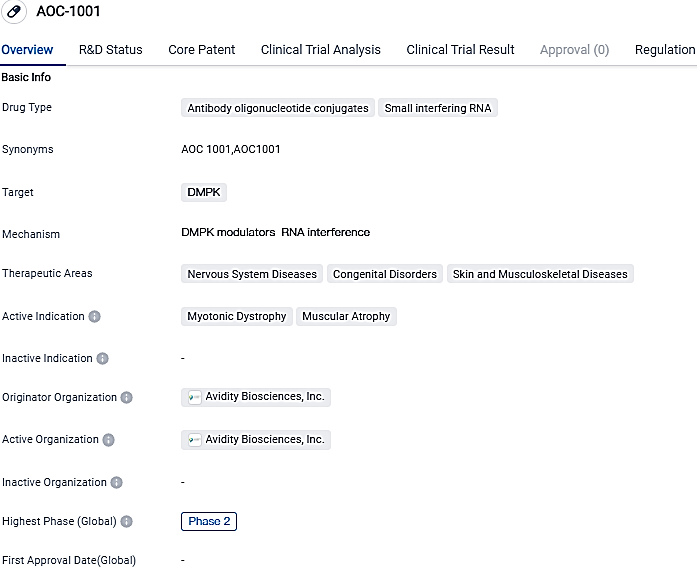
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
AOC 1001, the central clinical program from Avidity that uses its AOC platform, aims to target and tackle DM1, a frequently overlooked, progressive, and sometimes deadly neuromuscular disorder which currently lacks FDA-approved therapies. Key insights from the AOC 1001 data in both the MARINA® Phase 1/2 trial and its corresponding open-label extension study are set for discussion at the 28th Annual Congress of the World Muscle Society being held at Charleston, South Carolina.
"Looking at the latest set of data from AOC 1001 suggests not only an increasement in muscular power but also a positive impact on patient reported results, adding credence to the already positive topline data we have on enhancements in myotonia and mobility. We are impressed with AOC 1001's progressive outcomes across different operational endpoints," commented Nicholas E. Johnson, M.D., M.Sci., FAAN, a Vice Chair in neurology research and an associate professor at Virginia Commonwealth University.
Johnson carried on remarking, "The positive data from AOC 1001 efficacy studies and the reassuring long-term safety data provide us with confidence that AOC 1001 may be a promising treatment for DM1 patients, who urgently require effective therapies."
The new set of AOC 1001 data reveals improvements in other functional criteria, such as hand power, muscle strength, and patient reported outcomes. This enhances prior positive data which showed enhancements in strength, myotonia and movement. The latest safety data, collected from over 200 infusions which total roughly 46.2 patient-years of exposure, suggest that AOC 1001 holds a good safety profile with mainly mild to moderate side effects.
"Evidence from both MARINA and MARINA-OLE reinforce our trust in AOC 1001's potential to become an effective treatment for people affected by DM1, an egregious rare condition that currently doesn't have approved treatment methods. We're finalizing AOC 1001's Phase 3 trial plan and global regulatory pathway, and anticipate a preliminary glimpse at the efficacy data from MARINA-OLE in the first half of 2024," said Sarah Boyce, President and CEO of Avidity.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
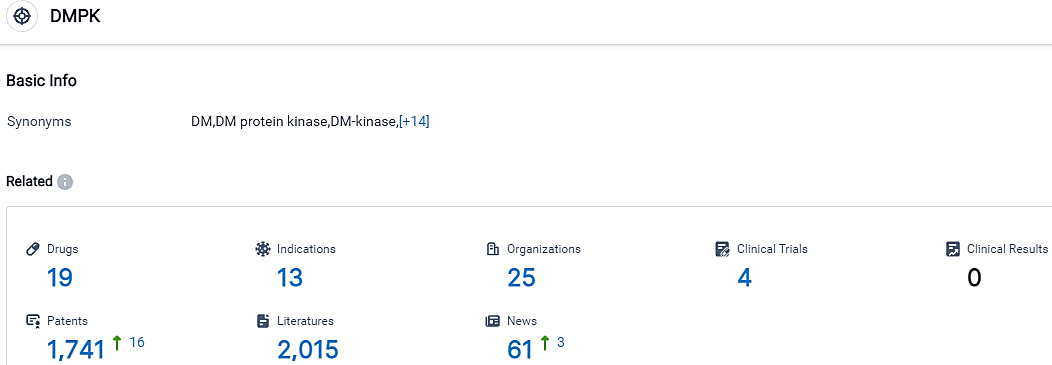
According to the data provided by the Synapse Database, As of October 17, 2023, there are 19 investigational drugs for the DMPK target, including 13 indications, 25 R&D institutions involved, with related clinical trials reaching 4,and as many as 1741 patents.
AOC-1001 exhibits potential for managing myotonic dystrophy and muscular atrophy. Its therapeutic action, which utilizes antibody oligonucleotide conjugates and small interfering RNA, implies a focused strategy for tackling the root causes of these disorders. The conferment of Fast Track and orphan drug statuses underscores the possible importance of AOC-1001 in fulfilling unmet healthcare requirements and addressing rare diseases.