Unveiling the FTO Retrieval of Multi-gene Elements within the AAV Domain
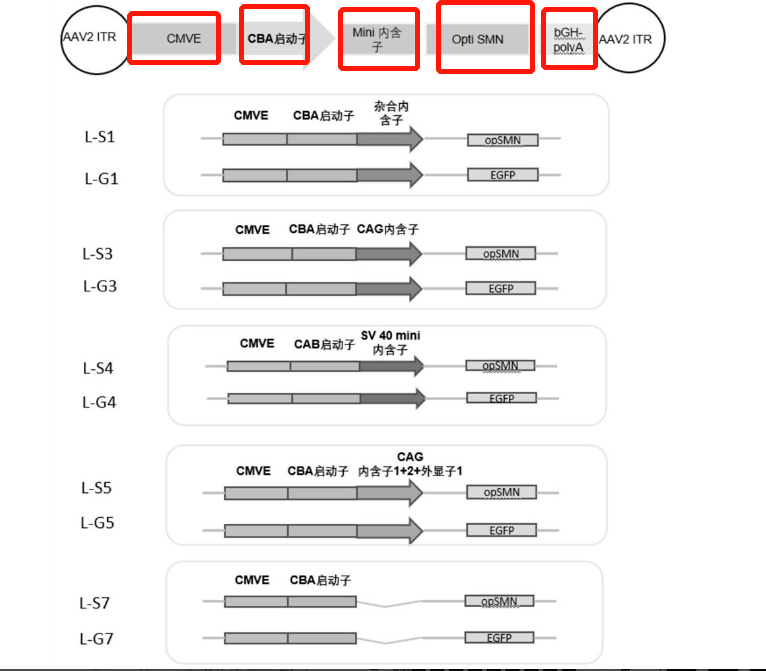
In the AAV field, common inventions involve the replacement and combination of expression-related gene regulatory elements. During FTO / novelty retrieval of sequences, it is generally not feasible to complete the retrieval with entire sequences. Instead, it's required to perform a combination search with major regulatory element sequences. The Patsnap Bio Sequence Database can satisfy the demand for such combination searches.
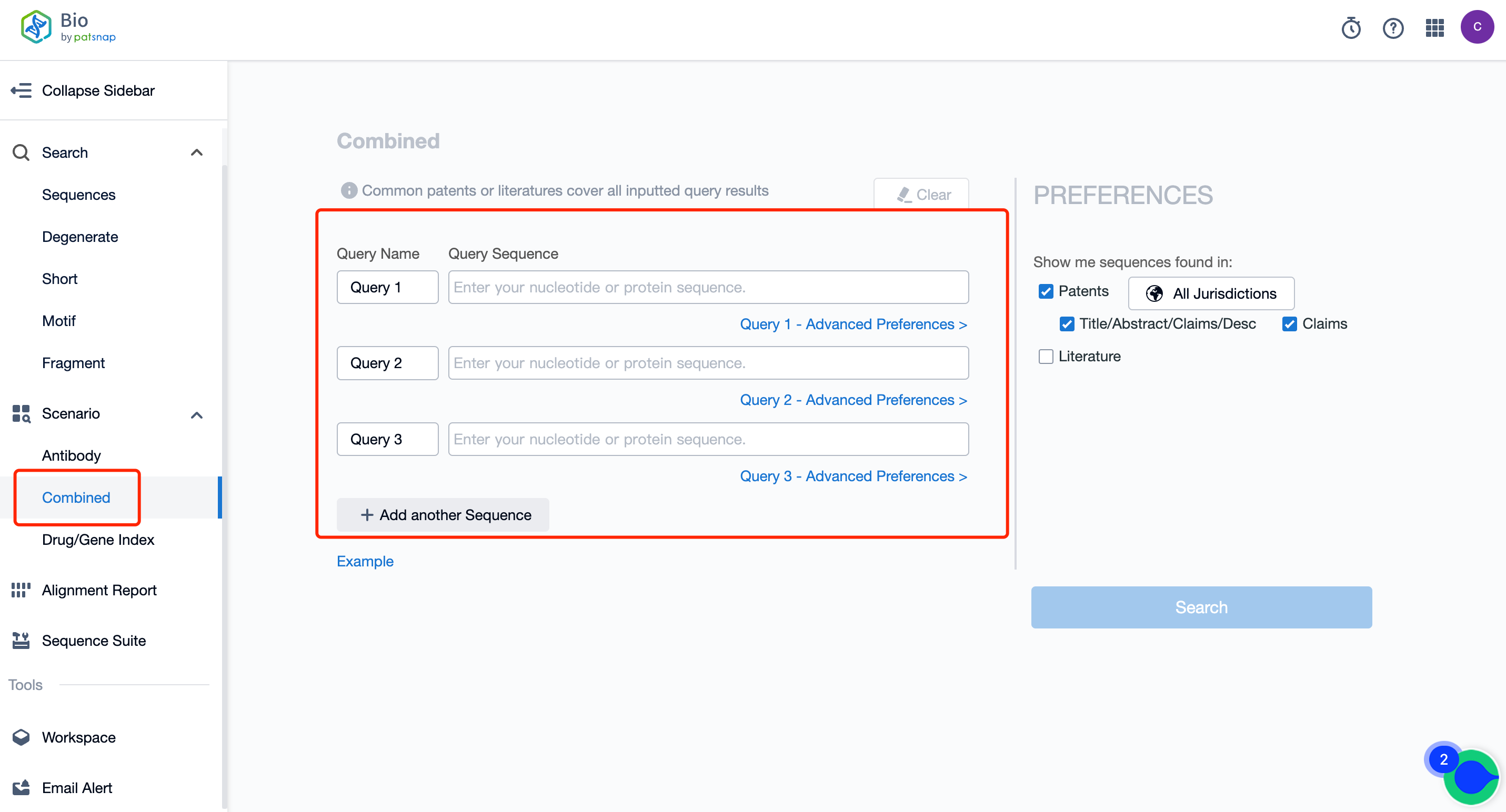
Firstly, click here to register a Patsnap Bio database account for free and go to the retrieval page. In the search options on the left, select " Combined" to get to the combination retrieval interface.
In the search box, input the sequences of the gene elements and target genes you want to query. If you want to search more than 3 sequences, click "Add another Sequence" (up to 6 sequences can be searched concurrently). For example, input the CMVE enhancer sequence, CBA promoter sequence, and target gene opSMN sequence, then choose patent or literature search.
It is important to note that Patsnap Bio is the most extensive sequence search platform for the Patsnap database. It incorporates AI with human-curated data for comprehensive handling of protein and nucleotide sequence data plucked from global patents, biological periodicals, and public repositories. Essential biological sequences are manually annotated, illuminating structural modifications to provide the most accurate sequence data and boost sequence retrieval efficiency.
Free registration is available for the Bio biological sequence database: https://bio.patsnap.com. Act now to expedite your sequence search tasks.