Oxitriptan Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs
Oxitriptan's R&D Progress
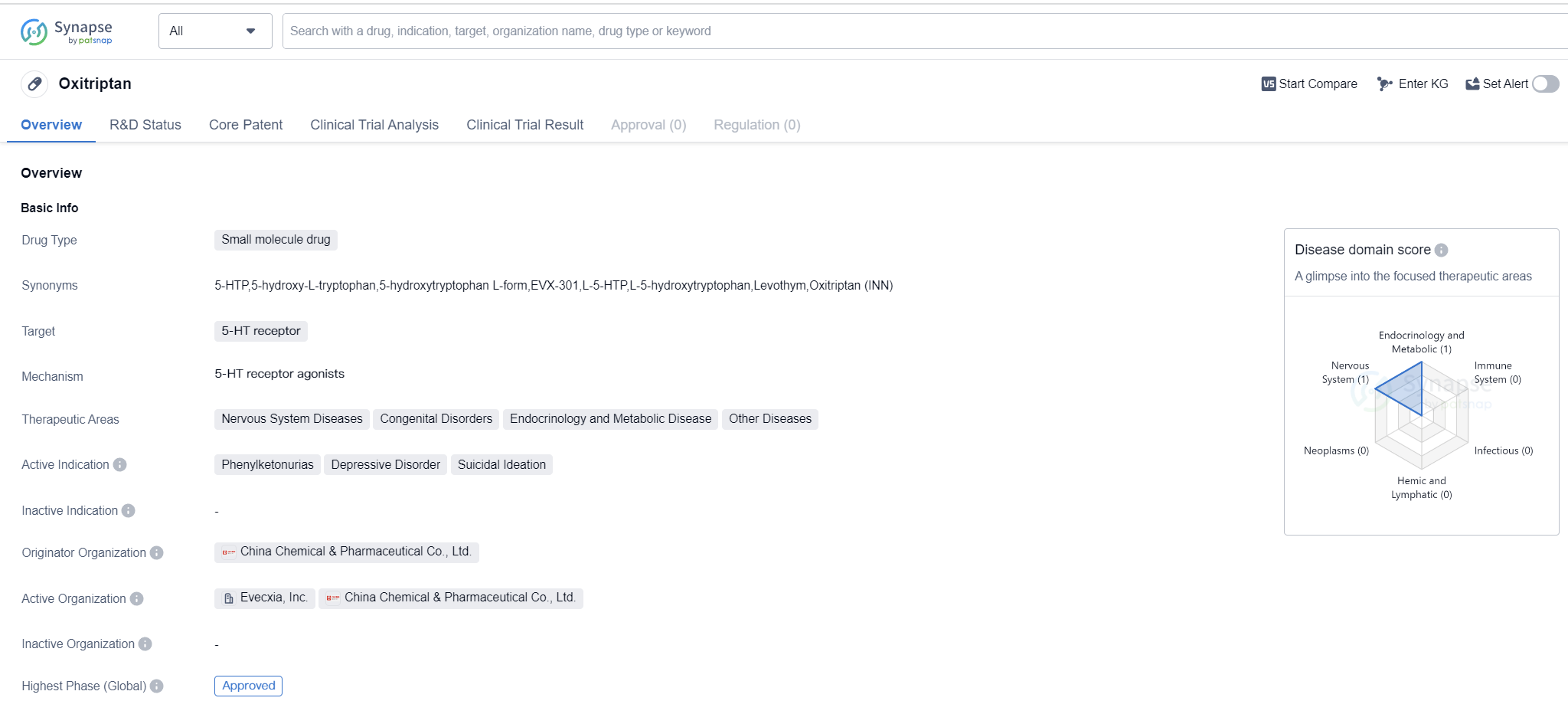
Oxitriptan is a small molecule drug that targets the 5-HT receptor. It has been approved for use in treating various therapeutic areas, including Nervous System Diseases, Congenital Disorders, Endocrinology and Metabolic Disease, and Other Diseases. The drug is specifically indicated for the treatment of Phenylketonurias, Depressive Disorder, and Suicidal Ideation.
Oxitriptan was developed by China Chemical & Pharmaceutical Co., Ltd., an originator organization based in China. The highest R&D phase of this drug is approved. This indicates that it has successfully undergone rigorous testing and evaluation to demonstrate its safety and efficacy.
The first approval of Oxitriptan took place in December 1995, with Taiwan Province being the first country/location to grant approval. This suggests that the drug has been available for use in Taiwan for over two decades.
Oxitriptan's targeting of the 5-HT receptor is significant as this receptor is involved in various physiological and pathological processes in the nervous system. By targeting this receptor, Oxitriptan may modulate serotonin levels, which can have an impact on mood regulation and other related functions.
The therapeutic areas for which Oxitriptan is indicated cover a wide range of diseases and disorders. Nervous System Diseases encompass a broad spectrum of conditions affecting the brain, spinal cord, and nerves. Congenital Disorders refer to conditions that are present at birth and may result from genetic or environmental factors. Endocrinology and Metabolic Disease involve disorders of the endocrine system, which is responsible for hormone production and regulation. Other Diseases likely include a variety of conditions that do not fall into the aforementioned categories.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for oxitriptan: 5-HT receptor agonists
5-HT receptor agonists are a class of drugs that bind to and activate the serotonin (5-HT) receptors in the body. Serotonin is a neurotransmitter that plays a crucial role in regulating various physiological processes, including mood, appetite, sleep, and pain sensation.
From a biomedical perspective, 5-HT receptor agonists can be used to modulate the activity of serotonin receptors, leading to a range of therapeutic effects. For example, certain 5-HT receptor agonists are used as antidepressants to alleviate symptoms of depression by increasing serotonin levels in the brain. They can also be used to treat migraines by constricting blood vessels and reducing inflammation.
There are several subtypes of 5-HT receptors, including 5-HT1, 5-HT2, 5-HT3, and so on. Each subtype has different functions and distributions in the body. Therefore, specific 5-HT receptor agonists may target specific subtypes to achieve desired therapeutic effects.
Overall, 5-HT receptor agonists are important pharmacological tools in biomedicine for modulating serotonin receptor activity and treating various conditions related to serotonin dysregulation.
Drug Target R&D Trends for oxitriptan
The 5-HT receptor, also known as the serotonin receptor, plays a crucial role in the human body. Serotonin is a neurotransmitter that regulates various physiological processes, including mood, appetite, sleep, and cognition. The 5-HT receptor is a target for many pharmaceutical drugs used to treat psychiatric disorders such as depression, anxiety, and schizophrenia. By modulating the activity of the 5-HT receptor, these drugs can help restore the balance of serotonin in the brain, alleviating symptoms and improving overall mental well-being. Understanding the role of the 5-HT receptor is essential for developing effective medications and therapies in the pharmaceutical industry.
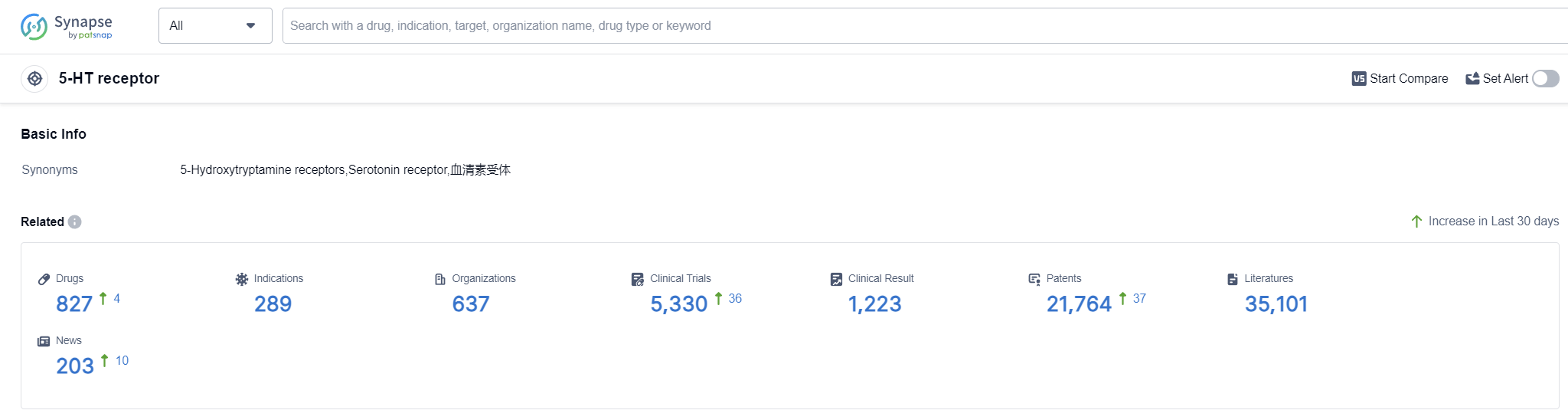
According to Patsnap Synapse, as of 12 Sep 2023, there are a total of 827 5-HT receptor drugs worldwide, from 637 organizations, covering 289 indications, and conducting 5330 clinical trials.
Based on the analysis of the target 5-HT receptor, it can be concluded that Johnson & Johnson, Sumitomo Chemical Co., Ltd., and Novartis AG are the leading companies in terms of R&D progress. Schizophrenia, migraine disorders, and depressive disorder are the indications with the highest number of approved drugs. Small molecule drugs are progressing rapidly, indicating intense competition in the market. The United States, China, and European Union are the countries/locations with the fastest development. China has shown significant progress in both approved and preclinical drugs. Overall, the target 5-HT receptor presents a competitive landscape with potential for future development in various indications and drug types.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Oxitriptan is a small molecule drug developed by China Chemical & Pharmaceutical Co., Ltd. It targets the 5-HT receptor and has been approved for use in treating various therapeutic areas, including Nervous System Diseases, Congenital Disorders, Endocrinology and Metabolic Disease, and Other Diseases. Its specific indications include Phenylketonurias, Depressive Disorder, and Suicidal Ideation. The drug received its first approval in Taiwan in December 1995 and has since been available for use in that location.