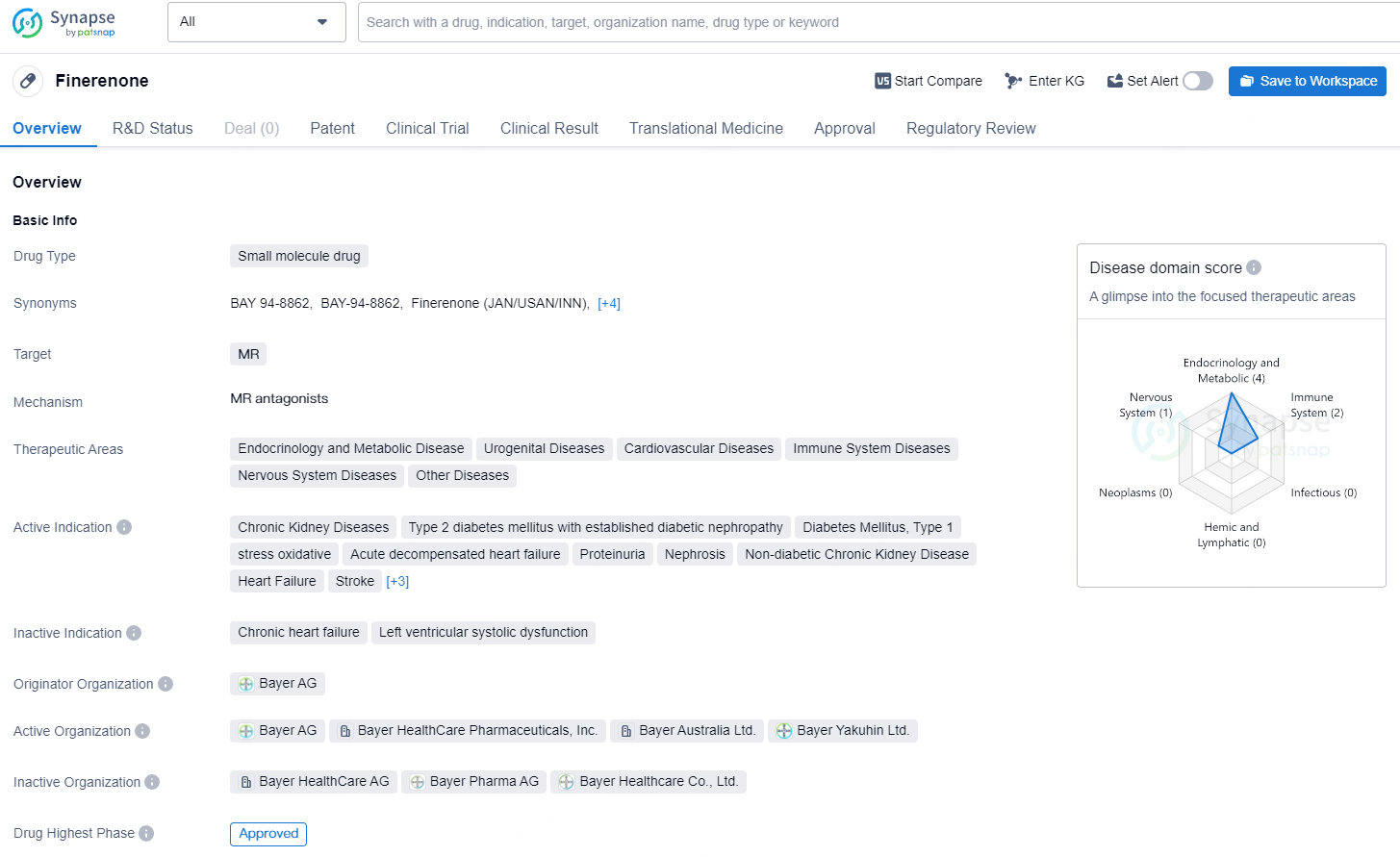
Bayer's FINEARTS-HF Phase III Study on KERENDIA® Shows Positive Results in Heart Failure Patients
Bayer revealed that FINEARTS-HF reached its main objective, demonstrating a statistically significant decrease in the combined incidence of cardiovascular death and overall heart failure events, which encompass HF hospitalizations or urgent HF consultations.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The Phase III cardiovascular outcomes trial, FINEARTS-HF, was a randomized, double-blind, placebo-controlled, parallel-group, and multi-center study assessing the efficacy and safety of KERENDIA (finerenone) in patients with heart failure (HF) with a left ventricular ejection fraction (LVEF) of 40% or higher. The parameter measures the percentage of blood ejected by the left ventricle per heartbeat.
In this trial, FINEARTS-HF, no new safety concerns were observed relative to those identified in earlier finerenone studies. KERENDIA has been approved to reduce risks like cardiovascular mortality, non-fatal myocardial infarction, HF hospitalization, sustained eGFR decline, and end-stage kidney disease in adults with chronic kidney disease (CKD) related to type 2 diabetes (T2D).
KERENDIA, a non-steroidal mineralocorticoid receptor antagonist (MRA), has demonstrated cardiovascular benefits in adults with CKD in T2D. The latest topline data indicates promising outcomes in a different patient population—those suffering from HF, independent of CKD in T2D. Bayer intends to present the FINEARTS-HF data at the European Society of Cardiology Congress 2024 in September and to discuss potential regulatory approval with the U.S. Food and Drug Administration.
FINEARTS-HF is part of the broader MOONRAKER program for KERENDIA, which aims to be one of the largest HF study programs to date, involving over 15,000 patients. Its goal is to provide a thorough understanding of KERENDIA’s impact on HF across various patient groups and clinical situations.
"Bayer is committed to advancing research and revolutionary treatments for diseases with significant unmet needs, including those with mildly reduced or preserved ejection fraction," stated Dr. Christian Rommel, Head of Research and Development at Bayer's Pharmaceuticals Division.
KERENDIA, a non-steroidal mineralocorticoid receptor antagonist, received approval from the U.S. Food and Drug Administration in July 2021 for reducing the risk of sustained eGFR decline, end-stage kidney disease, cardiovascular death, non-fatal myocardial infarction, and HF hospitalization in adults with CKD tied to T2D.
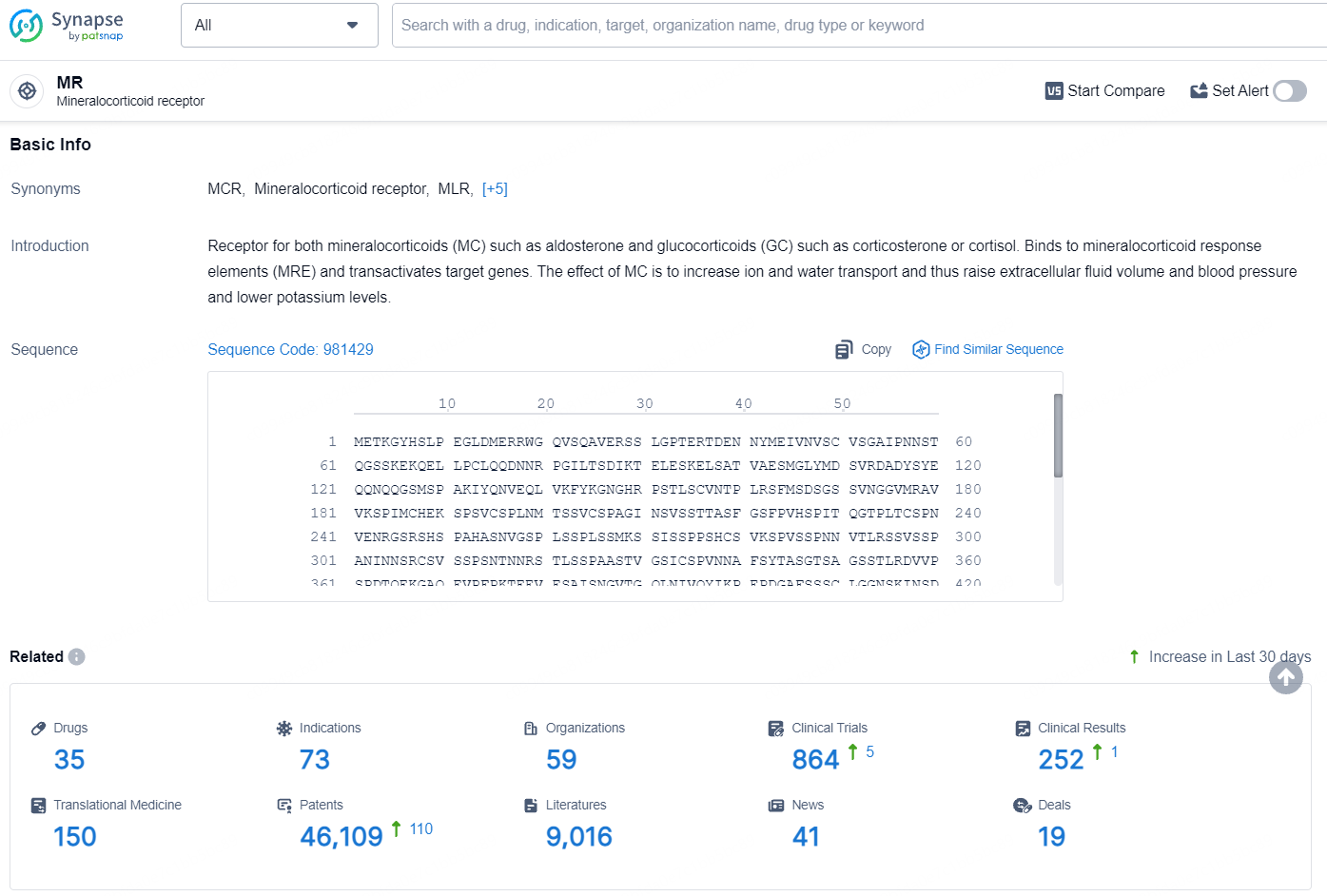
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 7, 2024, there are 35 investigational drugs for the MR targets, including 73 indications, 59 R&D institutions involved, with related clinical trials reaching 864, and as many as 46109 patents.
Finerenone is a small molecule drug developed by Bayer AG, with a broad range of therapeutic indications across multiple disease areas. Its approval in the United States in 2021 marks an important milestone for the treatment of chronic kidney diseases, diabetes mellitus, heart failure, and various other conditions. The drug's regulatory status under the Fast Track and Special Review Project reflects the recognition of its potential to address unmet medical needs and provide significant clinical benefits.