Johnson & Johnson withdraws from anti-epileptic drug, the 20-year collaborative development targeting mGluR2 comes to an end
Recently, the media has reported that Johnson & Johnson has decided to discontinue the clinical development of an anti-epileptic drug co-developed with their partner Addex Therapeutics targeting ADX71149 (JNJ-40411813) following the analysis of its Phase 2 clinical data. Johnson & Johnson stated that they are working closely with the clinical trial sites to notify the participating patients so they can seek alternative treatment options. This marks the end of the 20-year new drug collaborative research targeting mGluR2 receptors.
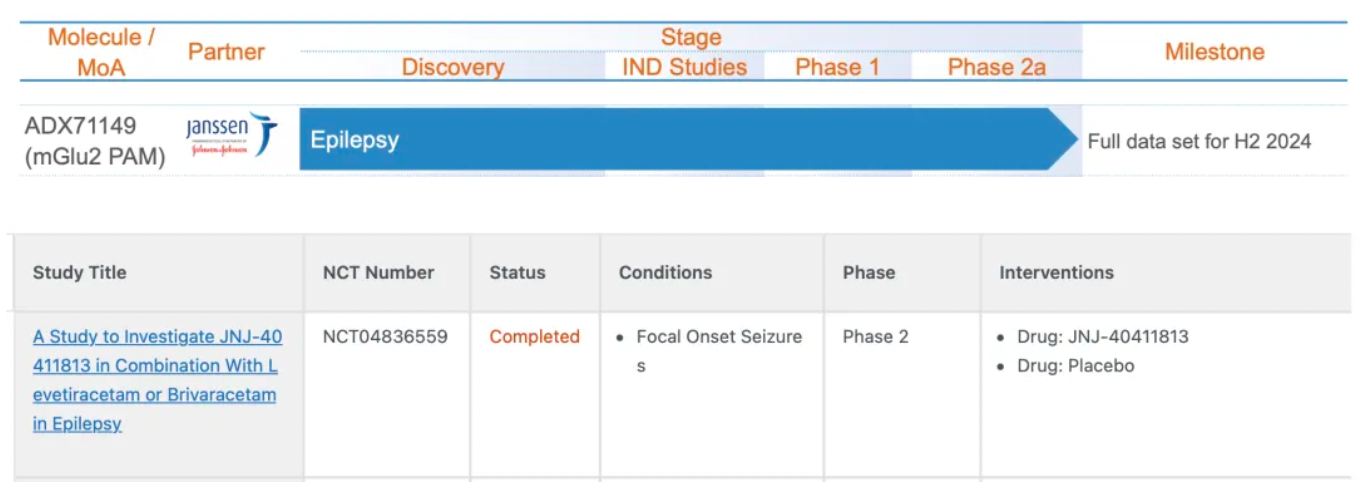
It is reported that ADX71149 (JNJ-40411813) is a small-molecule positive allosteric modulator (PAM) targeting mGluR2 with an activity value (pEC50) of 6.8. The investigational clinical indications include epilepsy, anxiety, and schizophrenia.

Johnson & Johnson started this collaboration with Addex in 2004 and in 2014, they first published early drug discovery data of this drug in "J Med Chem". In 2016, the clinical data from the depression Phase 2a study was released, which showed that while JNJ-40411813 was well-tolerated in patients, the results did not demonstrate its efficacy as adjunctive treatment for major depressive disorder (MDD) patients with significant anxiety symptoms within the studied dose range.

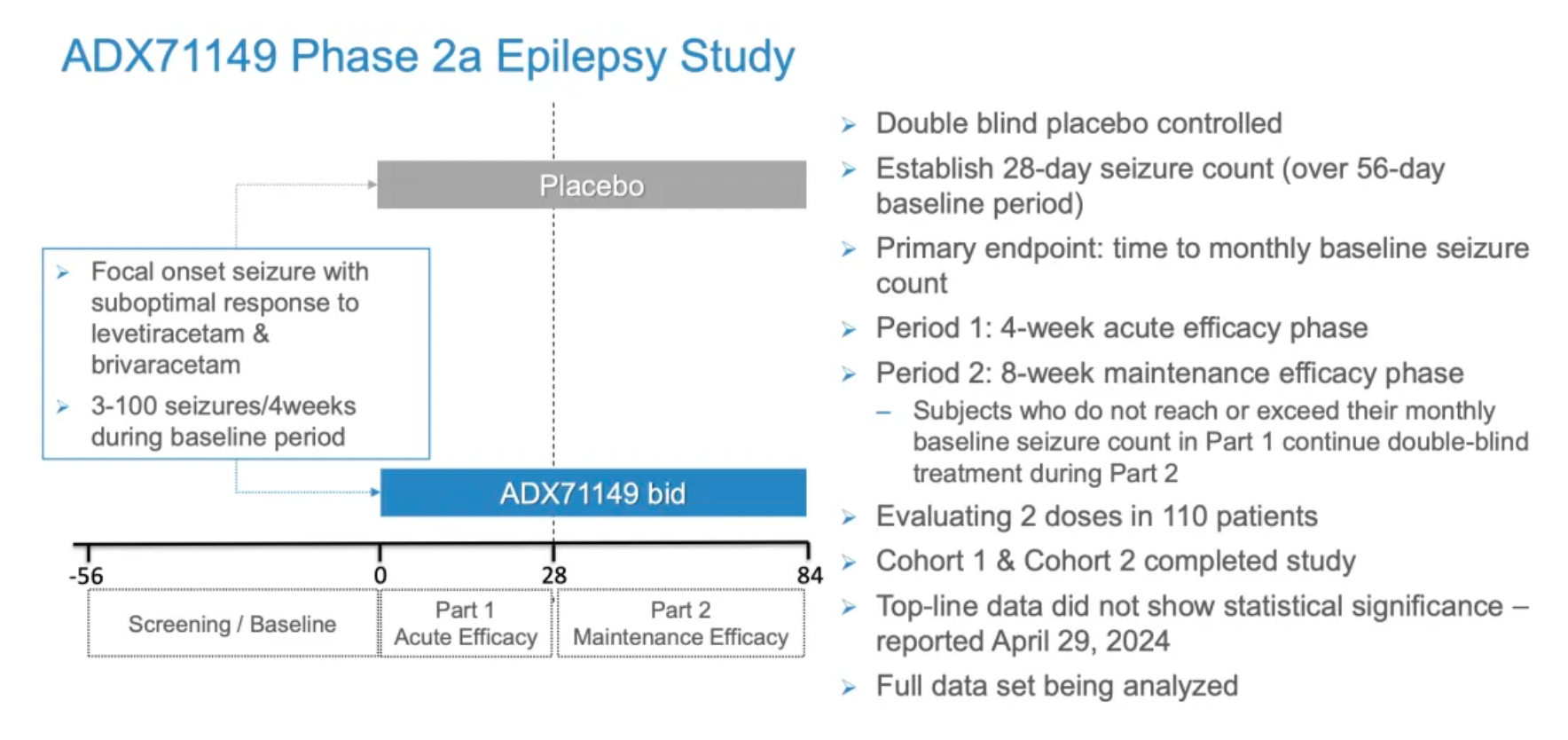
This multicenter Phase 2 clinical trial aims to evaluate the efficacy, safety, tolerability, and pharmacokinetics of ADX71149 as an adjunctive treatment in patients with focal onset seizures who have shown inadequate responses to two anti-epileptic drugs (Levetiracetam and brivaracetam). The primary objective of the study is to assess the efficacy of ADX71149 in combination with Levetiracetam or brivaracetam using the time endpoint of baseline seizure frequency.
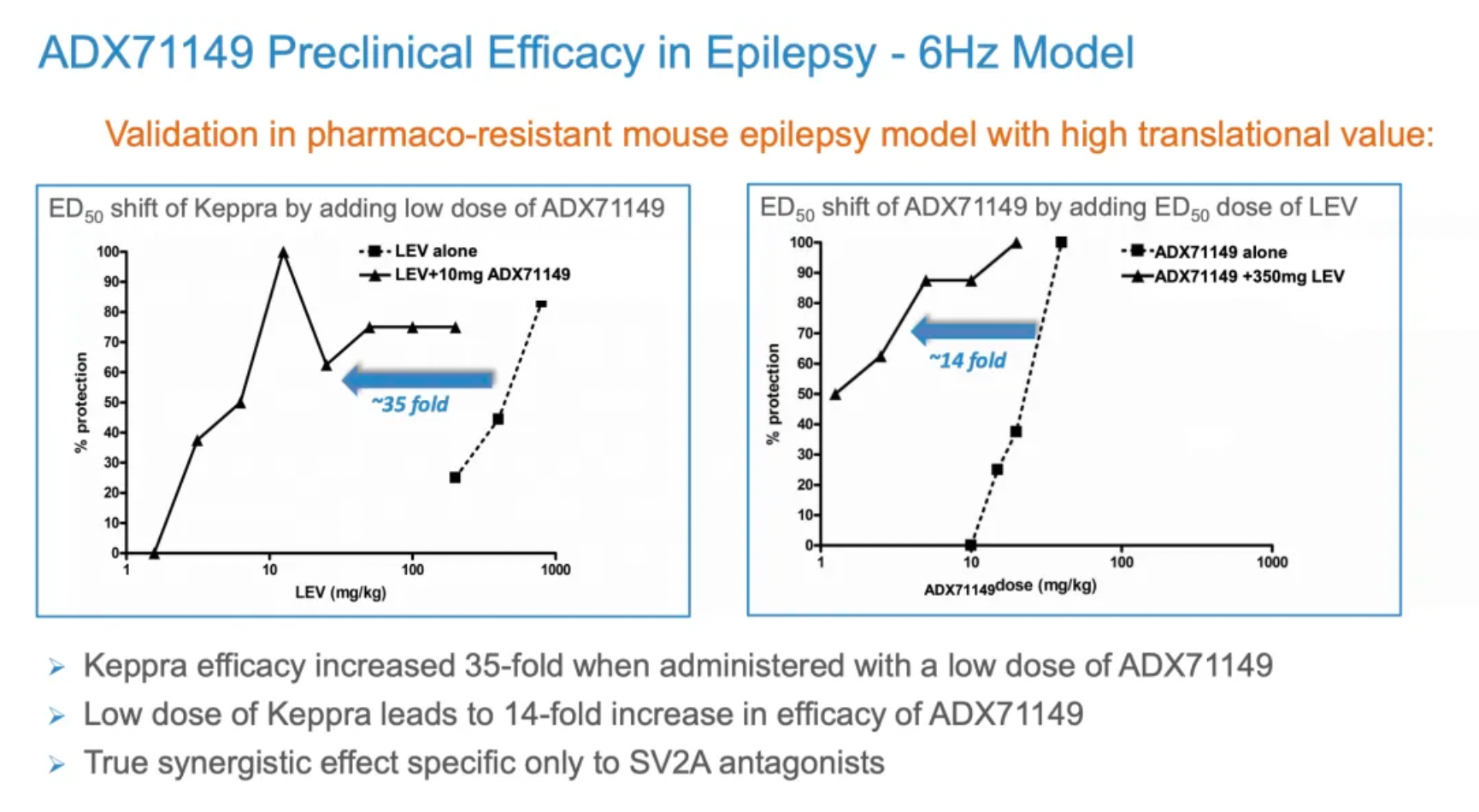
The following figure shows the research data of ADX71149 in preclinical epilepsy animal models.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!