Innovent and SanegeneBio Begin Early-Stage Trial for IBI3016 (AGT siRNA)
Innovent Biologics, Inc., an esteemed global biopharmaceutical enterprise focused on the development, production, and commercialization of premium medicines for oncology, autoimmune, cardiovascular, metabolic, ophthalmology, and other significant diseases, along with Sanegene Bio USA Inc., have announced the successful first dosing of a participant in a Phase 1 First-in-Human clinical trial of IBI3016.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
IBI3016 is an investigational siRNA drug candidate that targets angiotensinogen, aimed at treating hypertension. In preclinical trials, IBI3016 has been shown to notably decrease serum AGT protein levels and other related biomarkers in hypertensive cynomolgus monkeys, leading to significant and prolonged reductions in blood pressure, without any safety concerns such as hypotension.
This drug was developed using SanegeneBio’s next-generation proprietary siRNA technology platform, which increases drug potency and longevity while ensuring a good safety and tolerability profile. In December 2023, Innovent and Sanegene Bio entered into a strategic partnership to jointly develop IBI3016. Innovent also holds an exclusive option to license the future development, manufacturing, and commercialization rights of IBI3016.
The FIH study (NCT06501586) is a Phase 1 clinical trial designed to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of single ascending doses (SAD) of IBI3016 in both healthy volunteers and patients with mild hypertension, as a preliminary step towards further clinical development.
Dr. Lei Qian, Vice President of Clinical Development at Innovent Biologics, commented: “siRNA therapies offer long-acting effects and stable efficacy, making them highly promising for managing chronic conditions, especially in cardiovascular and metabolic diseases where long-term disease control is crucial. By combining SanegeneBio’s expertise in siRNA drug development with Innovent’s clinical development capabilities in CVM diseases, we have swiftly moved IBI3016 into clinical trials. We are dedicated to a thorough, science-based clinical development approach and will closely collaborate with SanegeneBio to advance IBI3016’s clinical development with high quality and efficiency, aiming to bring this innovative treatment to patients with hypertension and possibly other diseases.”
Dr. Yuyan Jin, Senior Vice President of Clinical and Non-Clinical Development at SanegeneBio, stated: “Globally, hypertension is a major unmet clinical need. IBI3016, as an innovative RNAi therapy, has shown excellent drug activity, sustained efficacy, and favorable safety and tolerability in preclinical studies. We look forward to continuing our close partnership with Innovent as we meticulously execute the clinical development plan for IBI3016 and strive for positive outcomes.”
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
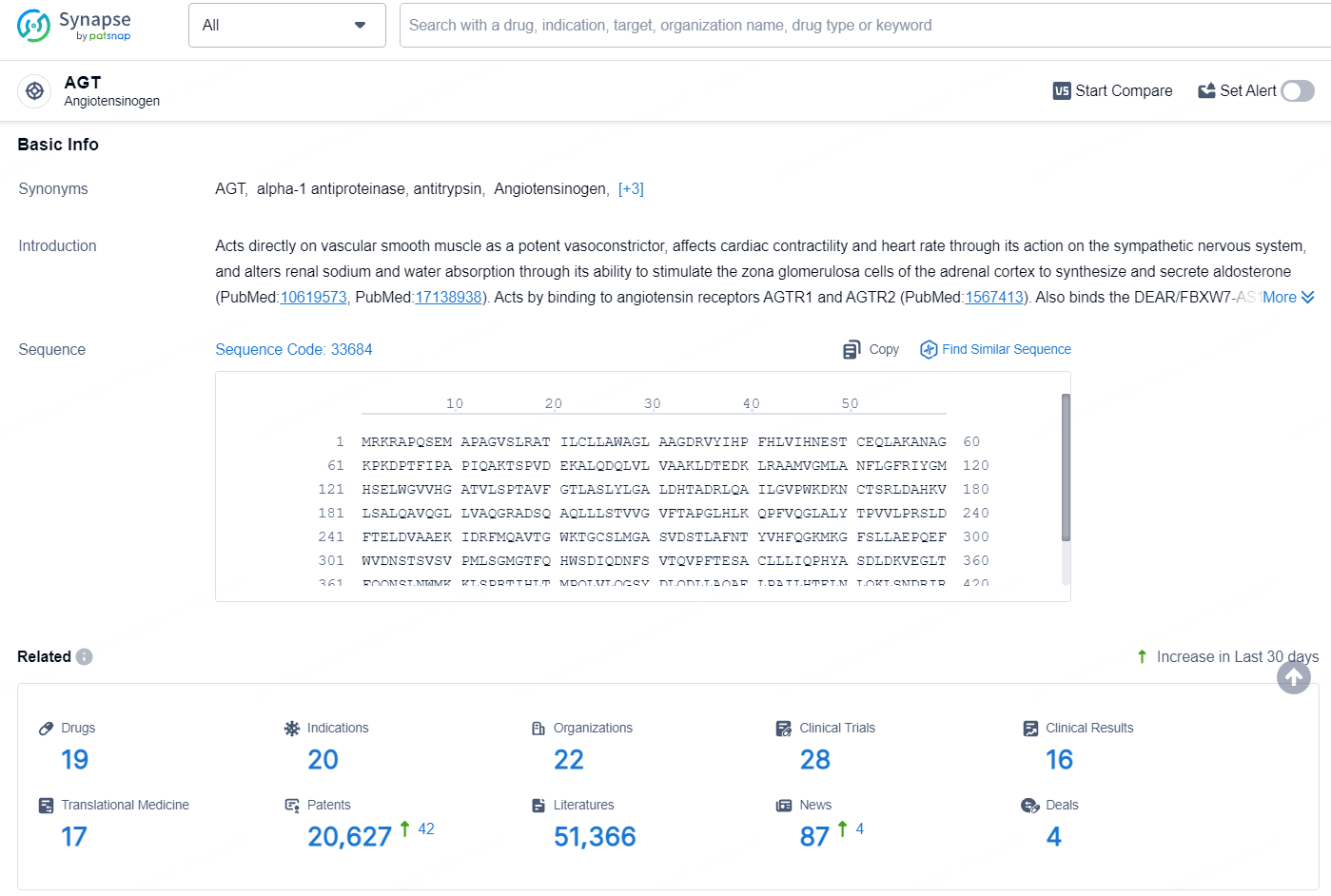
According to the data provided by the Synapse Database, As of August 7, 2024, there are 19 investigational drugs for the AGT target, including 20 indications, 22 R&D institutions involved, with related clinical trials reaching 28, and as many as 20627 patents.
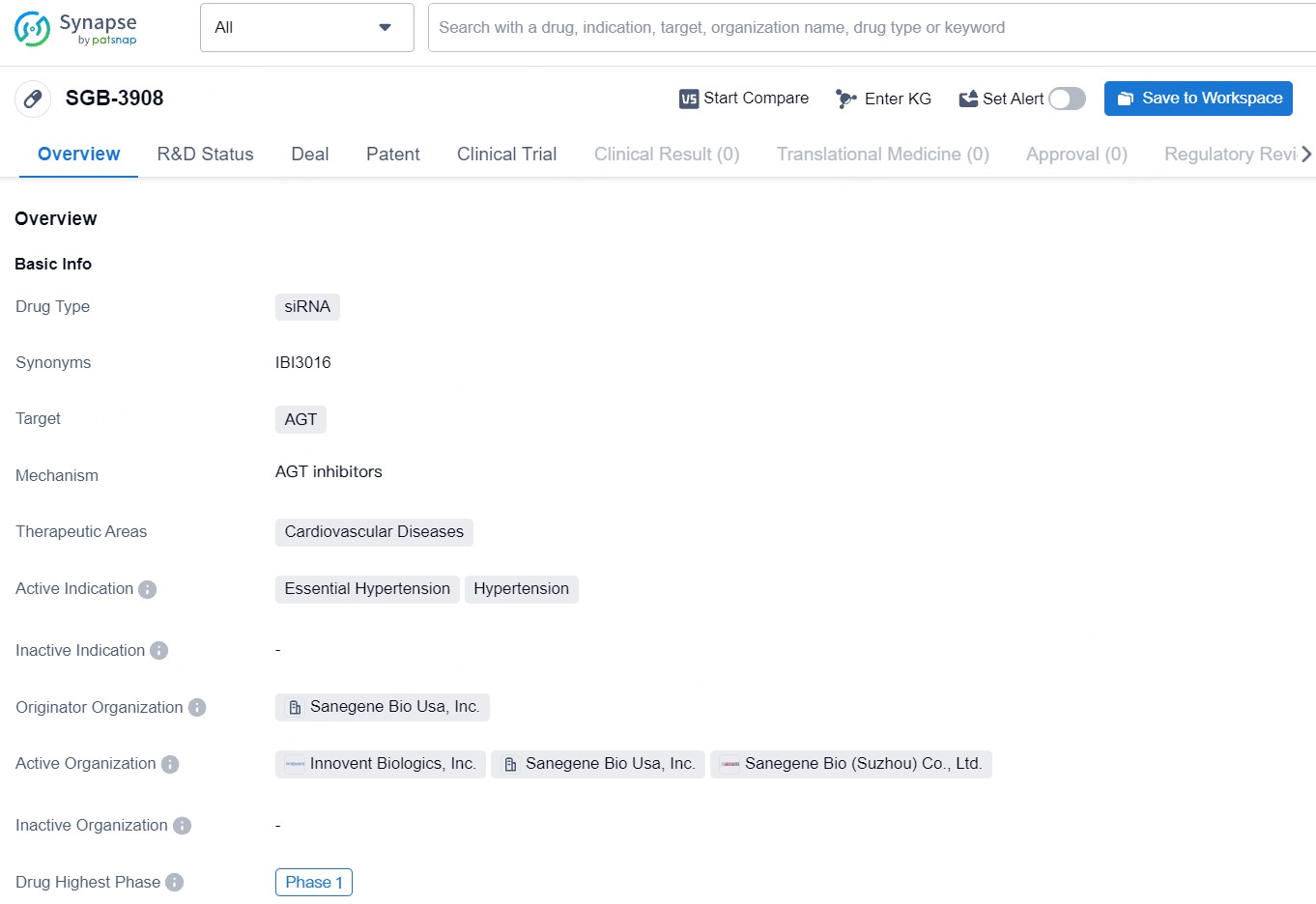
SGB-3908 is a siRNA-based drug targeting the AGT gene for the treatment of essential hypertension and hypertension within the cardiovascular disease therapeutic area. Its Phase 1 status suggests that it is in the initial stages of clinical development, and further research and testing will be necessary to determine its potential as a treatment option for these conditions.