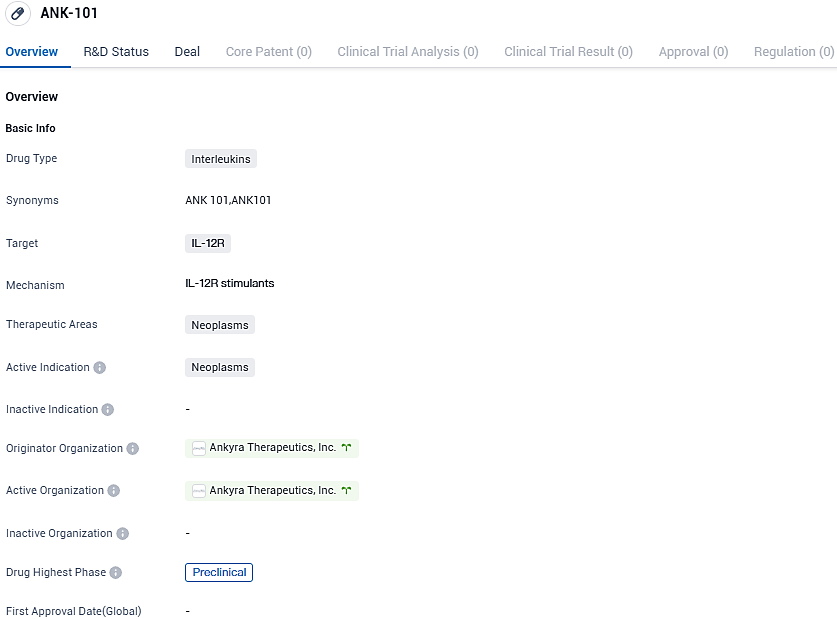
Ankyra Therapeutics reported encouraging preclinical outcomes using ANK-101, a new immune therapy, combined with cytotoxic chemotherapy in a head and neck cancer model
Ankyra Therapeutics, a budding clinical-stage biotech firm that leads the way in developing anchored immunotherapies for cancer treatment, revealed results from an early experimental study utilizing ANK-101, a therapeutic tethered to IL-12. This approach was combined with cytotoxic chemotherapy and immune checkpoint inhibition for the management of head and neck squamous cell carcinoma, simulated through mouse oral carcinoma.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The joint research work was performed by Ankyra Therapeutics along with the Center for Immuno-Oncology, which is a part of the National Institute of Health's National Cancer Institute Center for Cancer Research. The investigation's outcome will be showcased at the Society for Immunotherapy of Cancer Annual Meeting taking place in San Diego, California, from November 1-5, 2023.
Post trials of various combinations involving murine ANK-101, cisplatin, and anti-PD-1, whether individually or incorporated together, observations on tumor manifestation and mouse survival rates were recorded. The exhibited data indicated that one dose of mANK-101 corresponded with remarkable postponement in tumor development in the MOC1 model. The trials with anti-PD-1 and cisplatin, individually or in combination, exhibited minimum influence on tumor expansion.
Howard L. Kaufman, M.D., the CEO of Ankyra Therapeutics, stated: “ANK-101 shows hopeful early experimental outcomes, not just as individual therapy but even in conjunction with chemotherapy and checkpoint inhibitors.” He further emphasized that the data indicate that anchored IL-12 could potentially enhance the existing standard of care dealing with head and neck carcinoma, adding only slight additional toxicity.
Ankyra Therapeutics is advancing a unique category of anchored immunological cancer-targeting agents. Its principal asset includes IL-12 coupled with aluminum hydroxide. The IL-12 compound is formulated for retaining the effective IL-12 within the tumor milieu. Past preclinical trials showed IL-12 retention for even four weeks without showing systemic toxicity indications.Maximizing the therapeutic capacity while minimizing the systemic exposure and away from tumor-related side effects.
Leisha A. Emens, M.D., Ph.D., Senior Vice President of Translational Research of Ankyra Therapeutics expressed, “As we are gearing up to proceed to the clinical trials level, we are uplifted by these findings.” She asserted that in preliminary investigations, ANK-101 promotes immune activity within the tumor environment, making the tumor more susceptible to enhanced clinical benefits from the combined interventions of chemotherapy and PD-1 blockade.
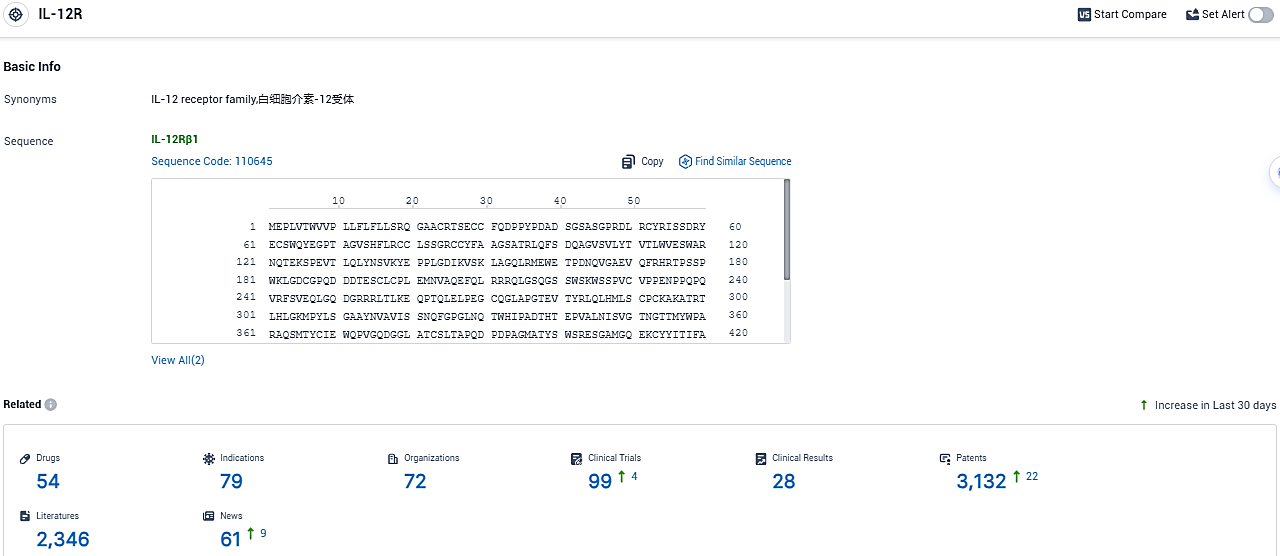
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 11, 2023, there are 54 investigational drugs for the IL-12R target, including 79 indications, 72 R&D institutions involved, with related clinical trials reaching 99, and as many as 3132 patents.
ANK-101 enables local delivery of functional IL-12 to the tumor microenvironment where it remains biologically active for several weeks but does not diffuse into the systemic circulation, thereby avoiding systemic toxicity. FDA and Health Canada have recently approved the company’s IND application for ANK-101: in early 2024, Ankyra plans to initiate a first-in-human Phase I clinical trial of ANK-101 as a single agent anchored immunotherapy in patients with advanced solid tumors who have failed standard of care treatments.