Analysis on the Clinical Research Progress of Fab Fragment
The Fab fragment, also known as the antigen-binding fragment, is the region of an antibody that can bind with antigens. The Fab fragment consists of a complete light chain and the VH and CH1 structural domains (Fd segment) of the heavy chain, with a molecular weight of approximately 50,000. Both the light and heavy chains contain a constant region and a variable region, with disulfide bonds linking the two chains.
As Fab possesses both an antigen-binding site and a part of the constant region, it not only has the same antibody-antigen affinity and excellent tissue penetration ability as a single-chain antibody (scFv), but also a more stable structure. Therefore, it plays a significant role in clinical diagnosis and treatment.
Compared with the complete IgG, the Fab fragment lacks the Fc region and, although it can selectively bind to the antigen, it does not precipitate. Due to its low autoimmunogenicity, it can't be recognized by living immune cells, thereby significantly reducing the likelihood of hypersensitivity reactions and enhancing the safety of a product. Fab-class antibody fragments have the characteristics of low molecular weight, strong tissue distribution specificity, low immunogenicity, and manipulability through gene engineering, making Fab an essential member in the field of pharmaceutical research. Fab-class drugs are widely applied in prevention, diagnosis, and treatment fields.
Fab Fragment Competitive Landscape
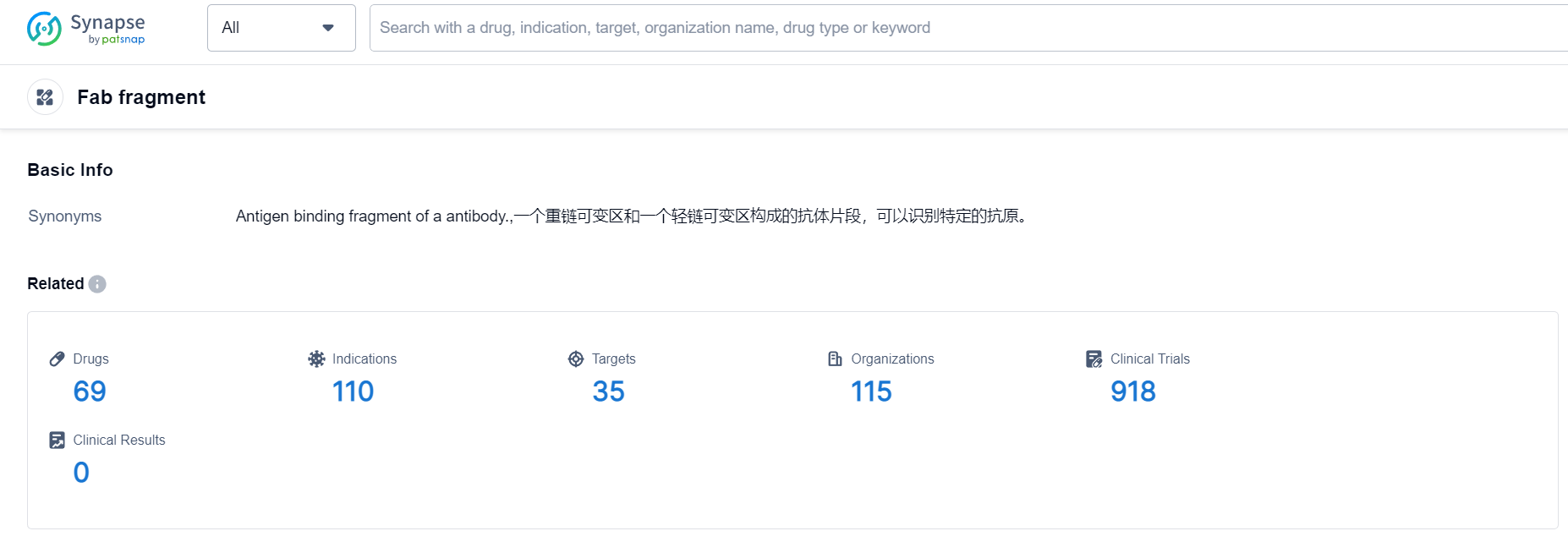
According to Patsnap Synapse, as of 8 Oct 2023, there are a total of 69 Fab fragment drugs worldwide, from 115 organizations, covering 35 targets, 110 indications, and conducting 918 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, targets, organizations, clinical trials, and clinical results related to this drug type.
Approved Fab Fragment Medicinal: Idarucizumab
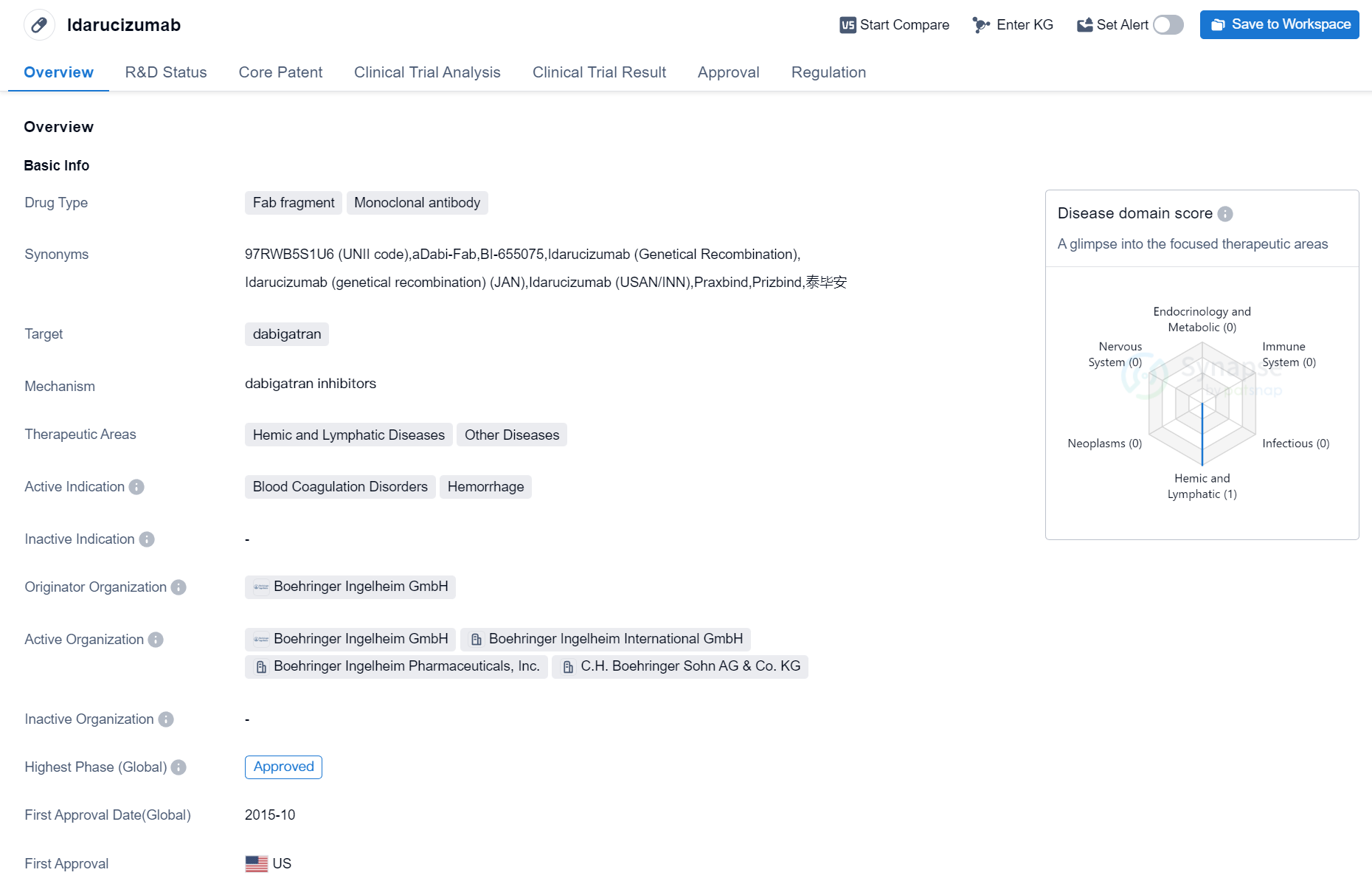
Idarucizumab is a drug that falls under the category of Fab fragment, monoclonal antibody in the field of biomedicine. It is designed to target dabigatran, a medication used to prevent blood clots. The therapeutic areas in which Idarucizumab is used include hemic and lymphatic diseases, as well as other diseases. The drug is primarily indicated for the treatment of blood coagulation disorders and hemorrhage.
Idarucizumab was developed by Boehringer Ingelheim GmbH, a pharmaceutical company. It has received approval for use in the highest phase globally, indicating that it has successfully completed clinical trials and met the necessary regulatory requirements.
The first approval of Idarucizumab took place in the United States in October 2015. This signifies that it was the first country to grant regulatory approval for the drug. Additionally, Idarucizumab is classified as an orphan drug, which means it is intended to treat rare diseases or conditions that affect a small number of individuals.
In summary, Idarucizumab is a Fab fragment, monoclonal antibody drug developed by Boehringer Ingelheim GmbH. It targets dabigatran and is used in the treatment of blood coagulation disorders and hemorrhage. The drug has received approval in global markets, with its first approval occurring in the United States in October 2015. Idarucizumab is classified as an orphan drug, indicating its intended use for rare diseases or conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Approved Fab Fragment Medicinal: Certolizumab Pegol
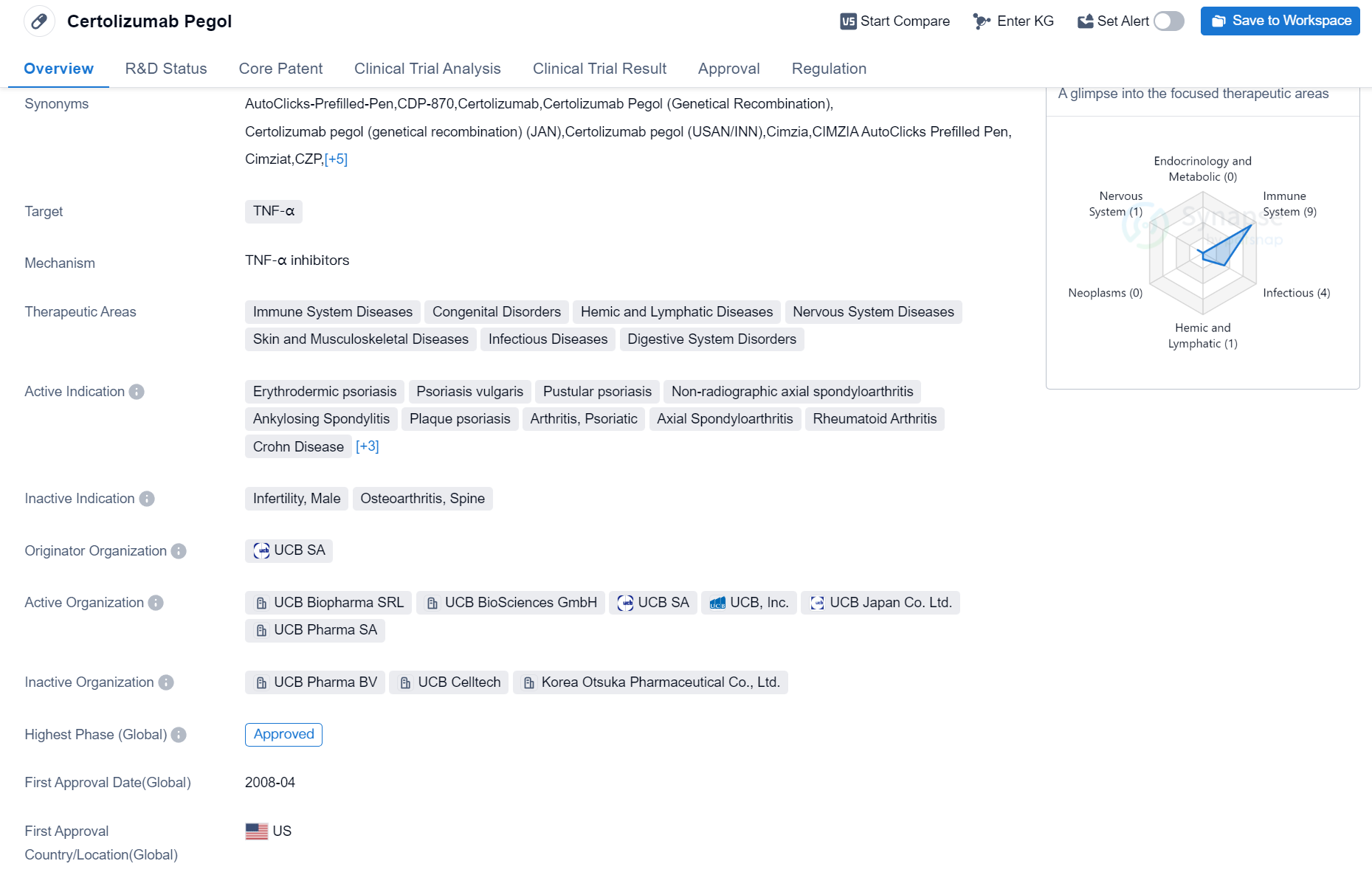
Certolizumab Pegol is a drug that falls under the category of Fab fragment, Monoclonal antibody. It specifically targets TNF-α, which is a protein involved in the immune system response. The drug has been approved for use in various therapeutic areas, including Immune System Diseases, Congenital Disorders, Hemic and Lymphatic Diseases, Nervous System Diseases, Skin and Musculoskeletal Diseases, Infectious Diseases, and Digestive System Disorders.
Certolizumab Pegol has been indicated for the treatment of several conditions, such as Erythrodermic psoriasis, Psoriasis vulgaris, Pustular psoriasis, Non-radiographic axial spondyloarthritis, Ankylosing Spondylitis, Plaque psoriasis, Arthritis (Psoriatic and Rheumatoid), Axial Spondyloarthritis, Crohn Disease, Psoriasis, Multiple Sclerosis, and Granulomatous Disease, Chronic.
The drug was developed by UCB SA, an originator organization in the pharmaceutical industry. It has received approval in multiple countries, with its first approval granted in the United States in April 2008. Certolizumab Pegol has undergone priority review, indicating its potential significance in addressing unmet medical needs or providing a substantial improvement over existing treatments.
As a Fab fragment, Monoclonal antibody targeting TNF-α, Certolizumab Pegol offers a promising therapeutic option for a wide range of diseases affecting various body systems. Its approval in multiple countries, including the United States, highlights its effectiveness and safety profile. The drug's diverse therapeutic areas indicate its potential to address multiple medical conditions, ranging from immune system disorders to digestive system disorders.
Certolizumab Pegol's approval in China further expands its reach and potential patient population. The drug's highest phase being approved globally signifies its advanced stage of development and successful clinical trials. With its first approval dating back to 2008, Certolizumab Pegol has established a significant presence in the pharmaceutical market, providing healthcare professionals and patients with a valuable treatment option.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Overall, Certolizumab Pegol's approval, therapeutic areas, and target specificity make it a noteworthy drug in the field of biomedicine. Its originator organization, UCB SA, has contributed to the advancement of pharmaceutical research and development, bringing innovative therapies to patients worldwide.