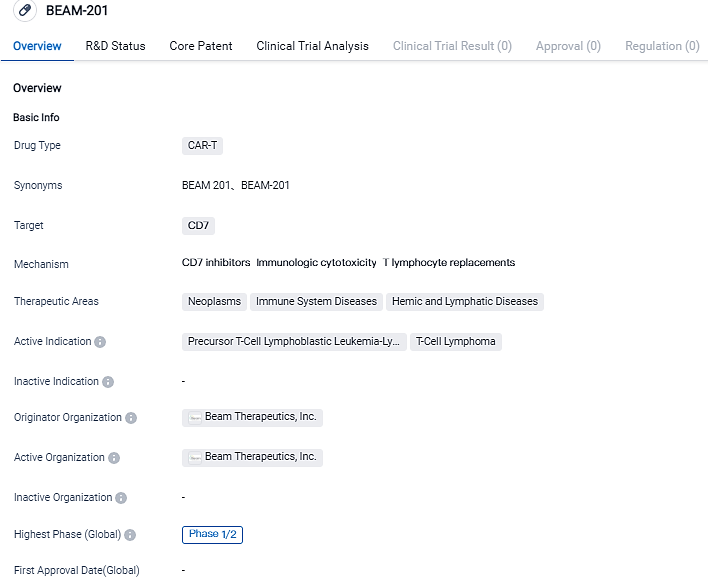
Beam Therapeutics reportes that the first patient dosed in the Phase 1/2 study of BEAM-201
Beam Therapeutics Inc., a firm in the biotechnology sector focusing on the creation of exact genetic treatments via base editing, declared that the first patient received BEAM-201 treatment. This new treatment is a quadruplex-edited allogeneic CAR-T cell therapy under investigation. The evaluation of BEAM-201 is underway in a clinical Phase 1/2 trial, with the aim to treat severe cases of relapsed/refractory T-cell acute lymphoblastic leukemia/T-cell lymphoblastic lymphoma, which is a harmful condition in both children and adults.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"With the first patient being treated with a therapeutic candidate from Beam and the first U.S. patient benefiting from a base editing therapy, we've reached a critical step forward for our organization, our dedicated scientists, and the patients we aim to assist," stated Beam's CEO, John Evans.
Beam-201 is being assessed in a multi-center, open-label Phase 1/2 trial related to safety and effectiveness in patients afflicted with relapsed/refractory T-ALL/T-LL. T-ALL/T-LL, a highly aggressive blood cancer resulting from malignant T cell precursor transformations, has limited therapeutic alternatives.
The first portion of the trial aims to evaluate safety, tolerability, and to identify the recommended Phase 2 dosage and lymphodepletion procedures. Important outcomes for the trial take into account treatment-induced and treatment-related side effects. The main efficacy objectives involve the proportion of patients that either yield complete or partial responses, those eligible for hematopoietic stem cell transplant, and those who reach a state of minimum residual disease negativity.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
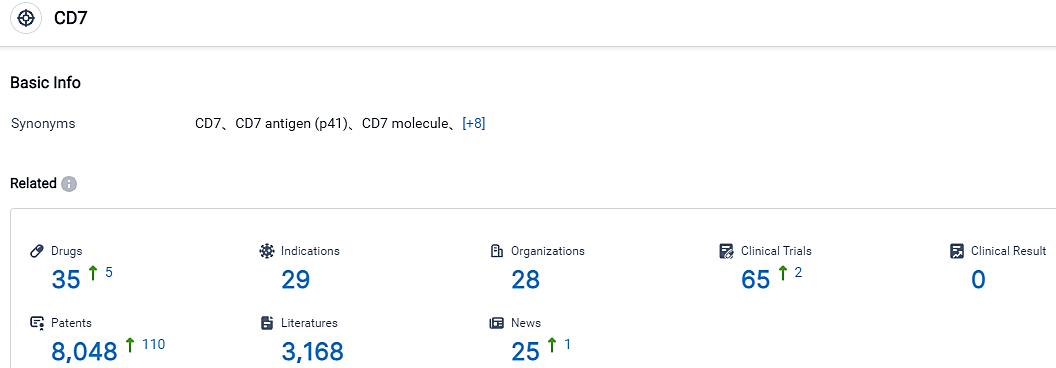
According to the data provided by the Synapse Database, As of September 7, 2023, there are 35 investigational drugs for the CD7 target, including 29 applicable indications,28 R&D institutions involved, with related clinical trials reaching 65,and as many as 8048 patents.
BEAM-201, anti-CD7 allogeneic chimeric antigen receptor T cell is currently undergoing clinical trials for the treatment of CD7+ relapsed/refractory T-cell acute lymphoblastic leukemia/T-cell lymphoblastic lymphoma. The intent behind multiplex base editing is to suppress the expression of CD7, TRAC, PDCD1, and CD52 genes. This strategy can potentially lessen fratricide, graft-versus-host disease, and exhaustion of CAR-T cells. Moreover, it allows BEAM-201 cells to avoid anti-CD52 lymphodepleting agents and to utilize an allogeneic cell resource.