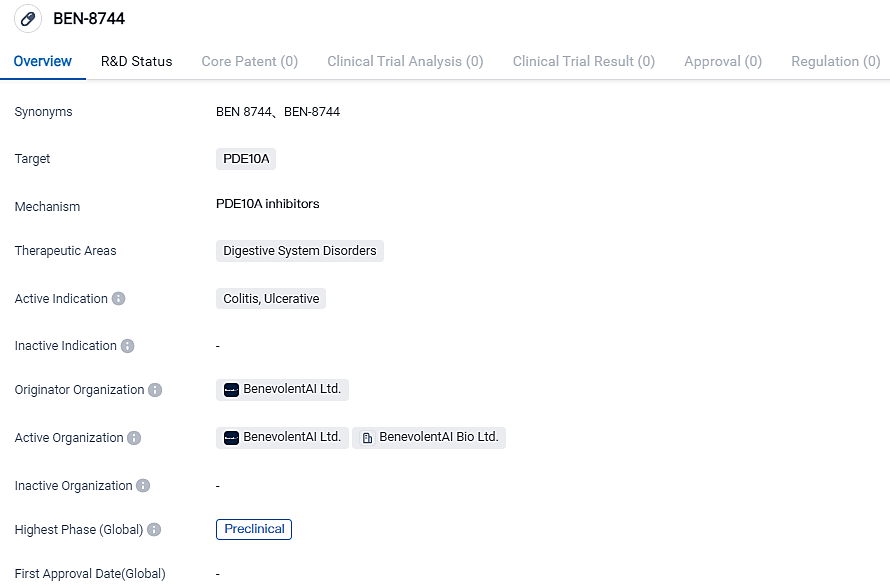
BenevolentAI's Doses First Participants of BEN-8744; Targets PDE10
BenevolentAI, a pioneer in creating innovative AI that boosts biopharma discovery, has now informed that its initial subjects have been administered in Phase I first-in-human trials of its oral phosphodiesterase 10 (PDE10) inhibitor, BEN-8744, designed to manage Ulcerative Colitis. They anticipate the prominent data from this analysis to be available in Q1 2024.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Dr. Anne Phelan, BenevolentAI's Chief Scientific Officer, noted: "UC is a medical condition where patients' needs have not been satisfactorily met, with ongoing remission proving to be a curveball. The launch of our Phase I study represents a significant stride toward addressing this multifaceted disease, making it the leading component of our clinical development projects. BEN-8744 reflects our forward-thinking approach by targeting an uncharted pathway, holding the promise of substantial differentiation from traditional standard-of-care treatments."
The CEO of BenevolentAI, Joanna Shields, commented: "Our AI-fueled drug discovery system identified PDE10 to be a unique target for UC, with no earlier, direct associations reported in scientific studies. BEN-8744 represents the potential of our tech platform to discover fresh opportunities for disease treatment."
About BEN-8744
BEN-8744 is an externally circumscribed small molecule PDE10 suppressor, currently under development as a potentially unprecedented treatment for Ulcerative Colitis (UC). Upon oral administration, it also holds potential to address other inflammatory bowel disease indications. BEN-8744 features a distinct therapeutic action for UC treatment, offering the chance for additional differentiation based on safety and effectiveness. PDE10 decreases the intracellular concentrations of the cGMP signaling molecule. With PDE10 suppression, restoration of cGMP levels is expected to serve an immediate anti-inflammatory and disease-altering advantage. BEN-8744 constitutes a solely owned entity in BenevolentAI's drug development pipeline.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
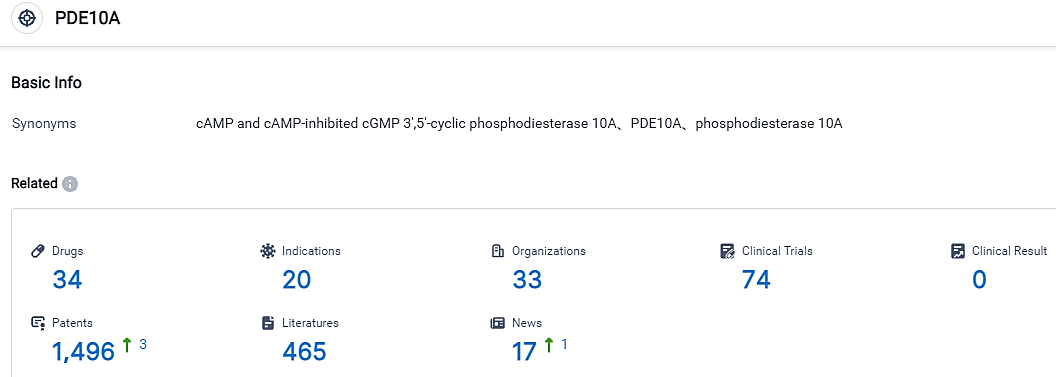
According to the information disclosed by the Synapse Database, as of September 1, 2023, there are 34 investigational drugs for the PDE10 target, including 20 applicable indications, 33 R&D institutions involved, with related clinical trials reaching 74, and as many as 1496 patents. UC is a persistent ailment that prompts inflammation and ulcer formation in the inner layer of the colon and rectum. It impacts roughly 0.4% of individuals living in the USA, and its severity reaches a moderate-to-high level in almost 31% of those diagnosed. However, between 20 to 40% of patients experiencing a moderate-to-intense form of UC fail to show a positive response to anti-TNF, which is the primary therapeutic approach. Current treatment methods, while necessary, may also trigger extreme adverse reactions.