PCSK9 inhibitors: A new promising lipid-lowering therapy
PCSK9 is a serine protease synthesized by the liver, which can bind to and degrade the low-density lipoprotein receptor (LDL-R), reducing LDL-R's clearance of low-density lipoprotein cholesterol (LDL-C) in the serum.
We all know that LDL-C is one of the four important indicators of clinical lipid testing; the higher its level, the greater the risk of Atherosclerotic Cardiovascular Disease (ASCVD). PCSK9 inhibitors can reduce blood lipid levels by blocking the binding of PCSK9 protein in the plasma to LDL-R (monoclonal antibody class) or directly blocking the synthesis of PCSK9 protein (siRNA class), thereby preventing the endocytosis and degradation of LDL-R, increasing the expression level of LDL-R on the cell surface, enhancing the reuptake of LDL-R to LDL-C, and reducing circulating LDL-C levels.
PCSK9 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 31 Aug 2023, there are a total of 91 PCSK9 drugs worldwide, from 113 organizations, covering 38 indications, and conducting 530 clinical trials. The analysis of the current competitive landscape and future development of target PCSK9 reveals that multiple companies are actively involved in R&D efforts.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
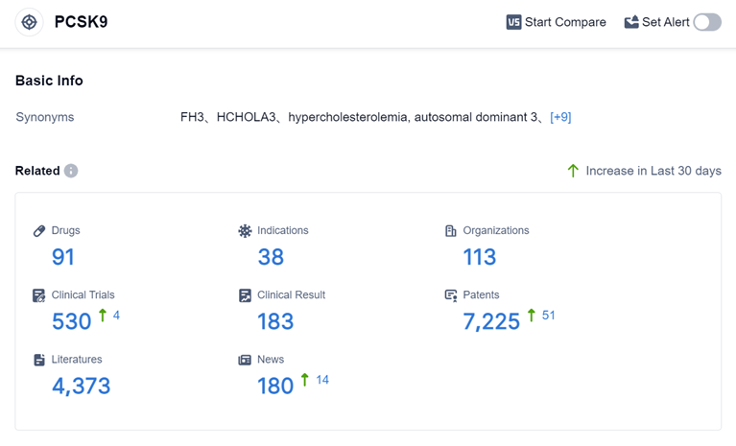
The indications for drugs targeting PCSK9 cover a wide range of cardiovascular diseases and lipid disorders. Monoclonal antibodies and small interfering RNA are the most prominent drug types. China, the United States, and the European Union are leading in terms of drug development, with China showing significant progress. The future development of target PCSK9 holds promise for the treatment of cardiovascular diseases and lipid disorders, with potential for innovative drugs and biosimilars.
Key Drug: Evolocumab
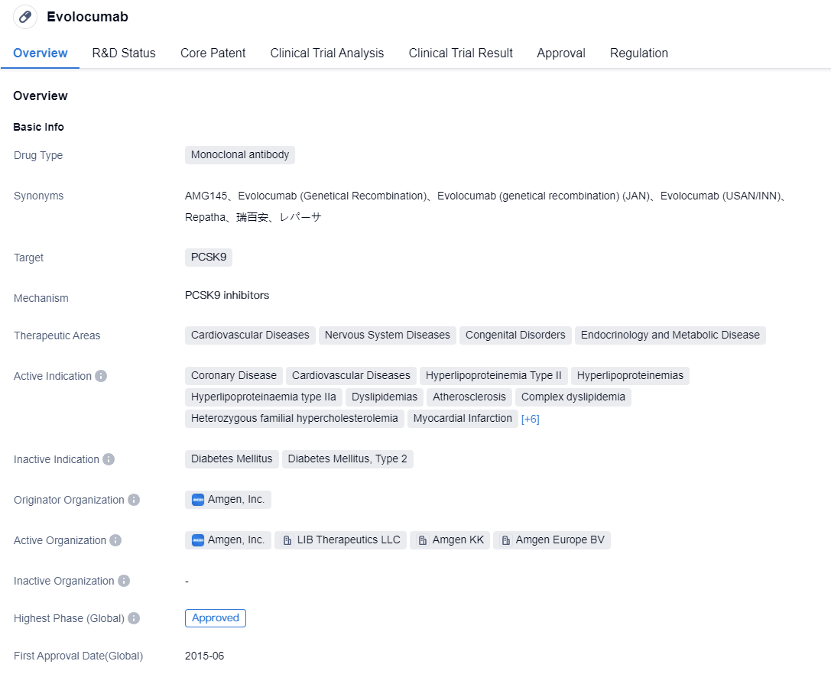
Evolocumab is a monoclonal antibody drug developed by Amgen, Inc. It targets PCSK9 and is used in the treatment of various cardiovascular and metabolic diseases. It has received approval in multiple countries, with its first approval granted in Norway in 2015.
Evolocumab, as a monoclonal antibody drug, has a high degree of selectivity and affinity for specific targets. By inhibiting the activity of PCSK9, it can effectively reduce LDL-C levels, thus reducing the risk of cardiovascular disease. In addition, Evolocumab can regulate lipid metabolism and improve the symptoms of diseases such as dyslipidemia and hypercholesterolemia. The drug has undergone extensive clinical trials and has reached the highest phase of approval globally and in China. The monoclonal antibody nature of Evolocumab allows for targeted therapy, potentially reducing side effects and improving treatment outcomes.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Inclisiran
Inclisiran is a small interfering RNA drug developed by Alnylam Pharmaceuticals, Inc. It targets PCSK9 and is indicated for the treatment of various cardiovascular diseases, congenital disorders, endocrinology and metabolic diseases, and other diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
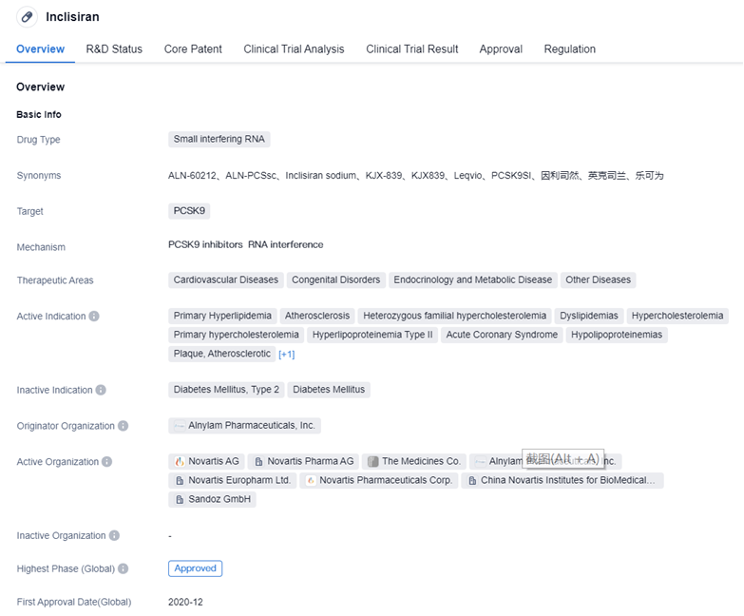
The key phase III clinical trials supporting the initial launch of Inclisiran in the EU and US are three in total: ORION-9, ORION-10, and ORION-11. These clinical studies enrolled a total of 3660 participants, of which 1728 patients with HeFH or ASCVD received Inclisiran treatment. After treatment with Inclisiran, these patients showed a 48% to 52% reduction in LDL-C levels on day 510 compared to baseline, compared to the placebo group, and the treatment effect remained relatively stable within 18 months. In terms of safety, the most common adverse events were mild to moderate injection site reactions, other common adverse events included joint pain, urinary tract infections, diarrhoea, and bronchitis; the Inclisiran group had fewer severe cardiovascular adverse events than the placebo group.




