CStone Pharmaceuticals' anti-PD-L1 monoclonal antibody, Sugemalimab, reached its primary endpoint in Phase 3 clinical trials
On August 29, 2023, CStone Pharmaceuticals announced that the Phase 3 study (GEMSTONE-303) of sugemalimab injection combined with first-line chemotherapy for the treatment of unresectable locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma with PD-L1 expression ≥5% has met its primary endpoint of overall survival (OS). Compared with the control group treated with placebo combined with chemotherapy, sugemalimab in combination with chemotherapy significantly prolonged patients' overall survival, with a difference that is statistically significant and clinically meaningful. So far, the GEMSTONE-303 study has met its prespecified dual endpoints of progression-free survival (PFS) and OS, as well as key secondary endpoints.
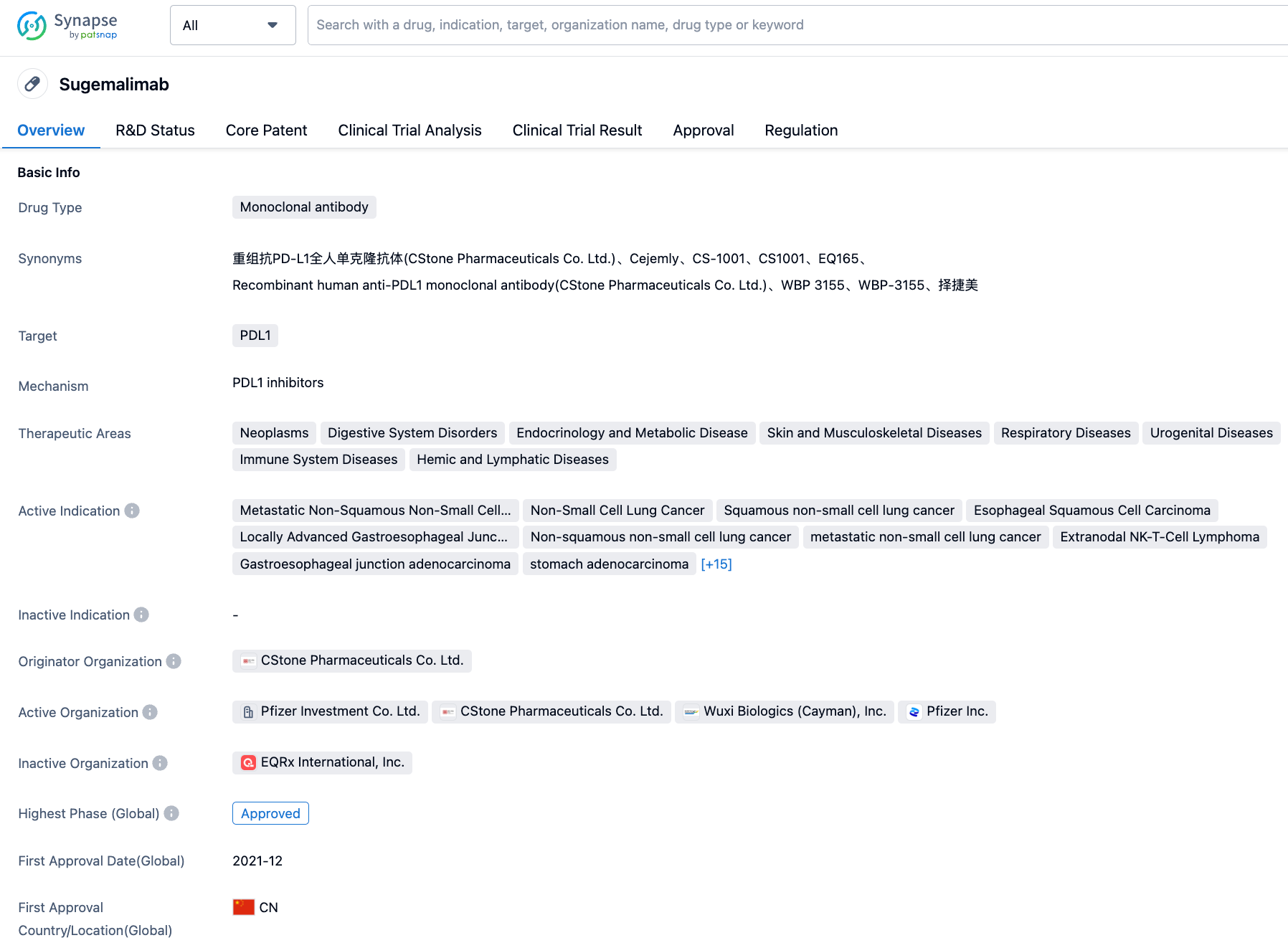
Sugemalimab is an anti-PD-L1 monoclonal antibody developed by CStone Pharmaceuticals. At present, sugemalimab has been approved in China for two indications: in combination with pemetrexed and carboplatin for first-line treatment of patients with metastatic non-squamous non-small cell lung cancer (NSCLC) who are epidermal growth factor receptor (EGFR) gene mutation-negative and anaplastic lymphoma kinase (ALK) -negative (as of December 2021), and in combination with paclitaxel and carboplatin for first-line treatment of patients with metastatic squamous NSCLC; it is also used for consolidation treatment after concurrent or sequential radiochemotherapy without disease progression in patients with unresectable stage III NSCLC (as of May 2022). Importantly, sugemalimab has been included in the 2022 edition of the CSCO NSCLC Diagnosis and Treatment Guide, recommended for first-line treatment in combination with chemotherapy for patients with stage IV non-driver gene non-squamous/squamous NSCLC, and as consolidation treatment following concurrent or sequential radiochemotherapy in patients with stage III NSCLC. In February 2023, the NMPA accepted the new indication marketing application for sugemalimab in combination with first-line chemotherapy for the treatment of unresectable locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma, which is currently under review.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
GEMSTONE-303 is a multicenter, randomized, placebo-controlled phase 3 registrational clinical trial, aimed at evaluating the efficacy and safety of sugemalimab in combination with CAPOX chemotherapy regime (oxaliplatin + capecitabine) as a first-line treatment for locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma that is inoperable and with PD-L1 expression ≥5%. The primary endpoints of this study are progression-free survival (PFS) and overall survival (OS) as assessed by researchers, with secondary endpoints that include PFS evaluated by Blinded Independent Central Review (BICR), and objective response rate (ORR) and duration of response (DoR) as assessed by researchers. In November 2022, GEMSTONE-303 study reached the primary endpoint of PFS, and interim analysis of OS showed a significant trend of benefit. Compared to the control group of placebo combination chemotherapy, sugemalimab combined with chemotherapy significantly improved PFS as assessed by researchers, and the difference has statistical significance and clinical relevance.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
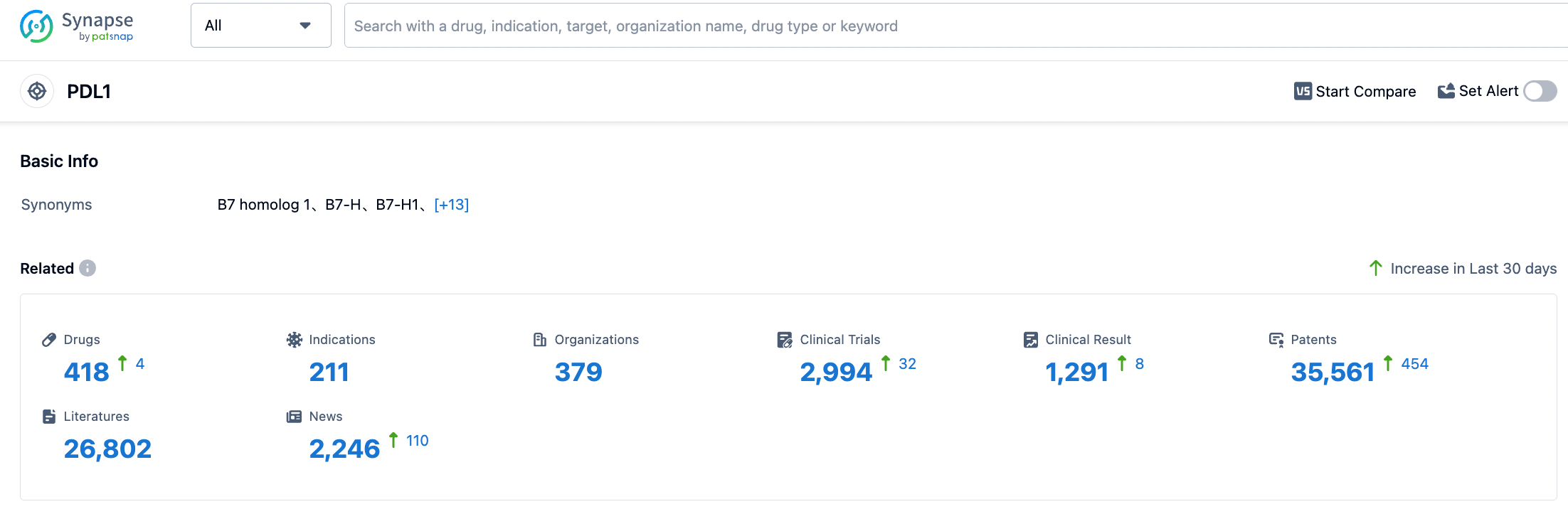
According to the information disclosed by Synapse, as of August 30, 2023, there are 418 drugs under research for the PD-L1 target, covering 210 indications, with 378 research institutions involved, related clinical trials amount to 2,993, and as many as 35,584 patents... In October 2020, BeiGene granted EQRx the development and commercialization rights for tislelizumab and anti-PD-1 monoclonal antibody CS1003 outside the Greater China region, with total collaboration amounting to $1.3 billion. EQRx has successively submitted applications for marketing authorization for first-line treatment of metastatic NSCLC with tislelizumab combined with chemotherapy to the Medicines and Healthcare products Regulatory Agency (MHRA) and the European Medicines Agency (EMA). There is hope that tislelizumab will successfully be launched in global markets.