Bicara Therapeutics Reveals New Phase 1/1b Results for Ficerafusp Alfa in HPV-negative Recurrent/Metastatic HNSCC
Bicara Therapeutics, a biotech firm focusing on clinical-stage development of innovative bifunctional therapies for individuals with solid tumors, revealed updated interim results from its ongoing, open-label Phase 1/1b dose expansion clinical trial of ficerafusp alfa (BCA101) during the 3rd Hawaii Global Summit on Thoracic Malignancies, scheduled for June 25-29, 2024.
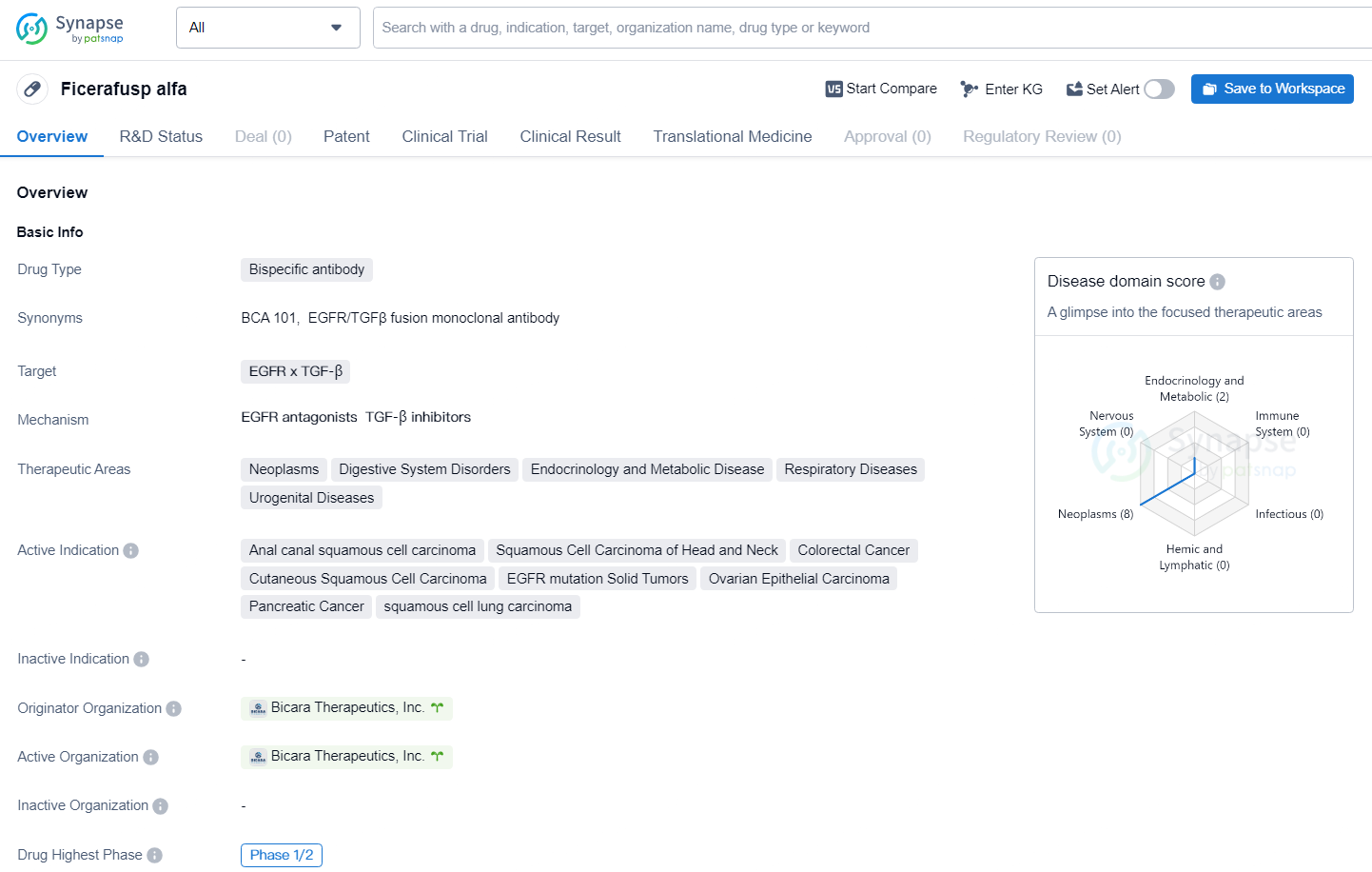
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Ficerafusp alfa is a dual-action antibody that targets two clinically validated molecules: a monoclonal antibody aimed at the epidermal growth factor receptor (EGFR) and a domain that attaches to human transforming growth factor beta (TGF-β).
In the Phase 1/1b clinical study, the combination of ficerafusp alfa and pembrolizumab demonstrated significant anti-tumor effects. The study showed an overall response rate of 64%, a complete response rate of 18%, and a median progression-free survival of 9.8 months in newly diagnosed human papillomavirus (HPV)-negative, recurrent/metastatic head and neck squamous cell carcinoma. The treatment was also well tolerated.
"Ficerafusp alfa has the capability to exert strong anti-tumor effects by simultaneously inhibiting cancer cell survival and proliferation via EGFR, as well as counteracting immunosuppressive TGF-β signaling within the tumor microenvironment. This dual action can lead to sustained responses and enhanced survival," stated Claire Mazumdar, Ph.D., MBA, CEO of Bicara Therapeutics.
"Considering these promising results, we plan to launch a pivotal Phase 2/3 study of ficerafusp alfa in combination with pembrolizumab for frontline treatment of HPV-negative R/M HNSCC. We are also enthusiastic about exploring the potential of ficerafusp alfa in other populations of HNSCC patients and various squamous cell tumors, where dual inhibition of EGFR and TGF-β is biologically justified," Claire Mazumdar further commented.
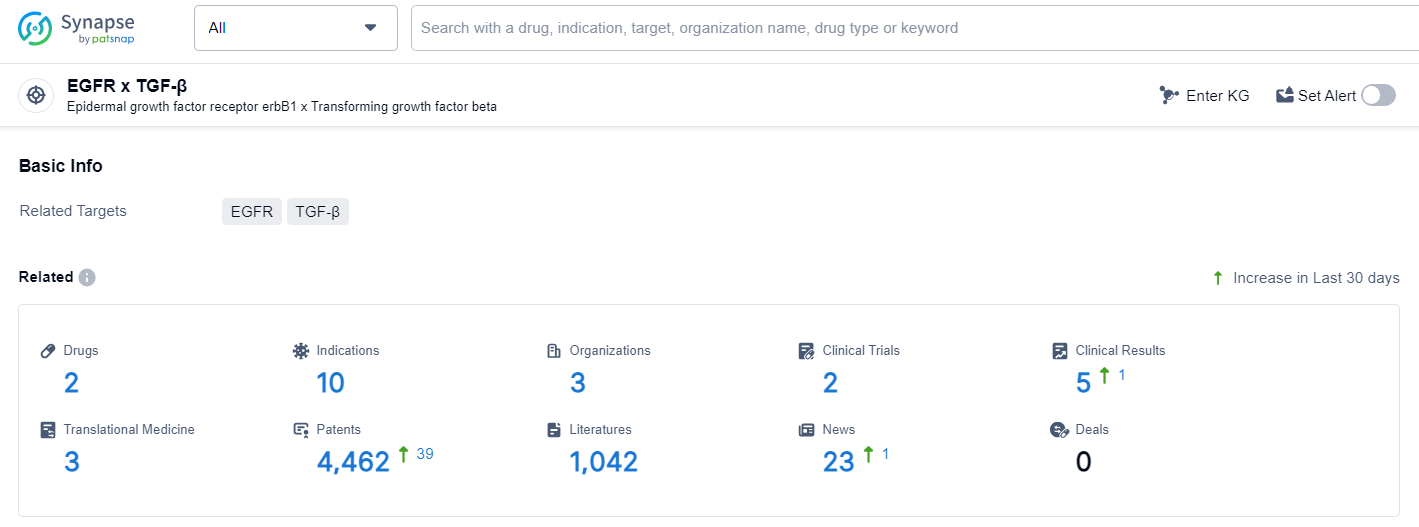
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 2, 2024, there are 2 investigational drugs for the EGFR and TGF-β target, including 10 indications, 3 R&D institutions involved, with related clinical trials reaching 2, and as many as 4462 patents.
Ficerafusp targets EGFR x TGF-β for the treatment of various cancers and related diseases. The drug has reached Phase 1/2 in its global development, and its potential to address unmet medical needs in the specified therapeutic areas makes it a promising candidate for further clinical development and potential commercialization.