Meitheal Pharmaceuticals Boosts Biologics Range through Exclusive Licensing Deal for YUSIMRY®, a Humira® Biosimilar
Meitheal Pharmaceuticals, Inc., an entirely integrated biopharmaceutical enterprise situated in Chicago, dedicated to the development and marketing of generic injectables, fertility treatments, biologics, and branded products, revealed today that it has secured exclusive commercial rights in the United States for YUSIMRY(adalimumab-aqvh), a biosimilar to Humira (adalimumab). This acquisition was achieved through an exclusive license and supply agreement with its parent company, Hong Kong King-Friend Industry Co., Ltd.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"The licensing of YUSIMRY represents a significant milestone for Meitheal, reinforcing our presence in the biosimilars sector and offering us a valuable asset with strong growth potential in the years ahead," stated Tom Shea, CEO of Meitheal Pharmaceuticals. "Leveraging our capabilities in research, manufacturing, and commercialization, Meitheal is well-equipped to improve patient access to top-quality biosimilars like YUSIMRY, while fostering its clinical and commercial progress."
HKF has secured global rights to YUSIMRY through an asset purchase deal with Coherus BioSciences, Inc. This agreement allows HKF to acquire nearly all assets associated with YUSIMRY, including developmental and regulatory outcomes, in return for an upfront payment of $40 million in cash. The asset purchase agreement and Meitheal’s exclusive licensing deal with HKF are now effective, pending standard conditions and approvals.
"Adalimumab is essential for many Americans managing autoimmune diseases, and we are excited to continue providing YUSIMRY, a more cost-effective alternative to Humira, to patients at a reasonable and sustainable cost," commented Brian McCarthy, Meitheal's Senior Vice President of Specialty. "With adalimumab biosimilars gaining momentum, YUSIMRY is well-placed to satisfy the ongoing need for affordable treatment options, driven by significant macro trends and strategic investments in its production and commercialization."
In recent times, Meitheal and its parent firm HKF have made numerous investments to enhance the company's biologics portfolio and capabilities. The new, exclusive licensing agreement for YUSIMRYintroduces a fifth biosimilar and the first in-market biosimilar to Meitheal’s lineup. HKF and its affiliated entities have invested over $300 million in capital and R&D in the past years to ensure a sustainable product supply, including a $30 million investment in a monoclonal antibody drug substance facility.
"We are delighted to have acquired the rights to this vital treatment option and believe that Meitheal is optimally positioned to provide affordably priced YUSIMRY to U.S. patients in need," said Eric Tang, President of HKF. "We remain committed to strategic investments in the crucial biosimilars market and look forward to an ongoing partnership with Meitheal to promote accessible healthcare solutions."
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
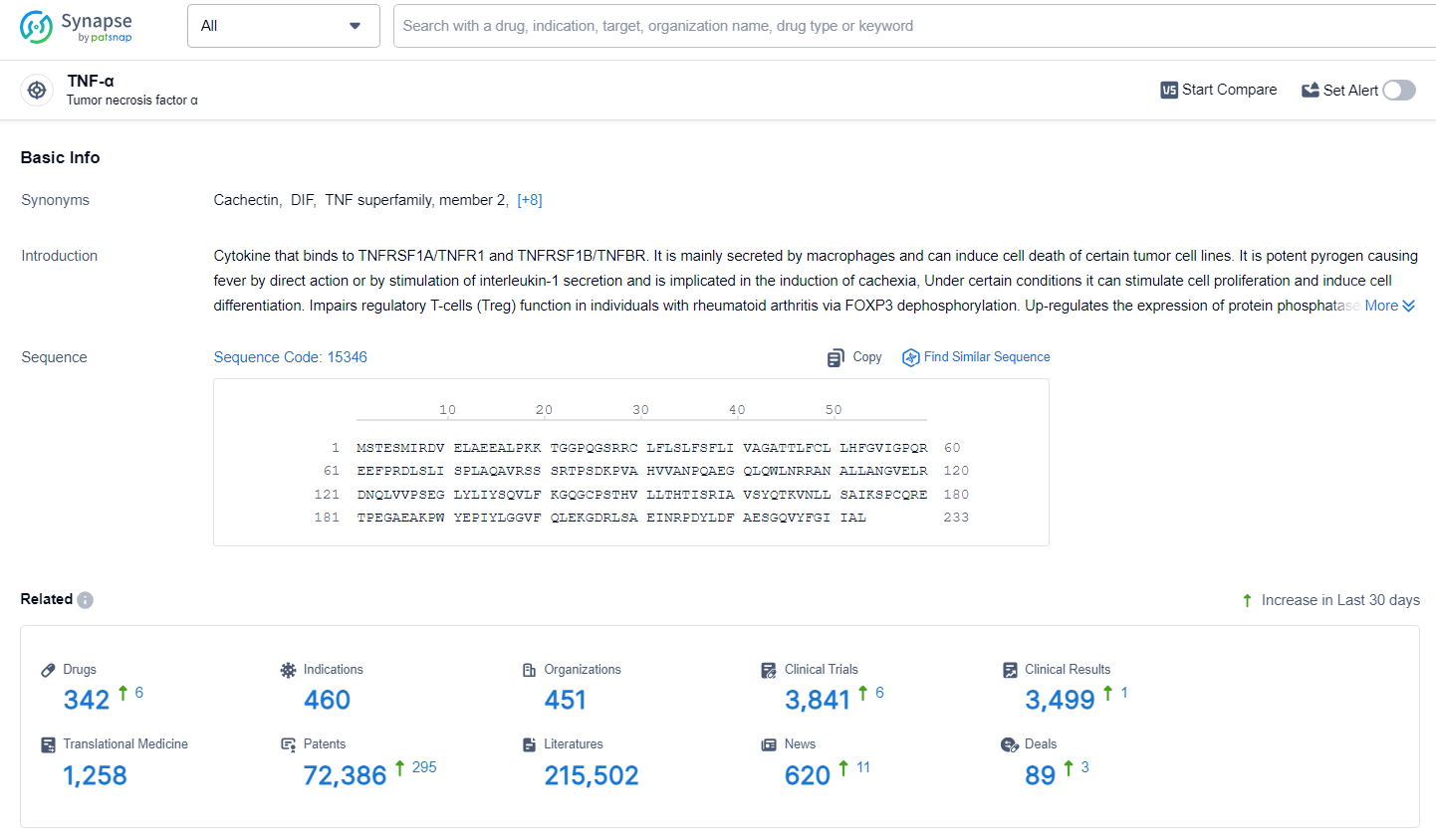
According to the data provided by the Synapse Database, As of July 2, 2024, there are 342 investigational drugs for the TNF-α target, including 460 indications, 451 R&D institutions involved, with related clinical trials reaching 3481, and as many as 72386 patents.
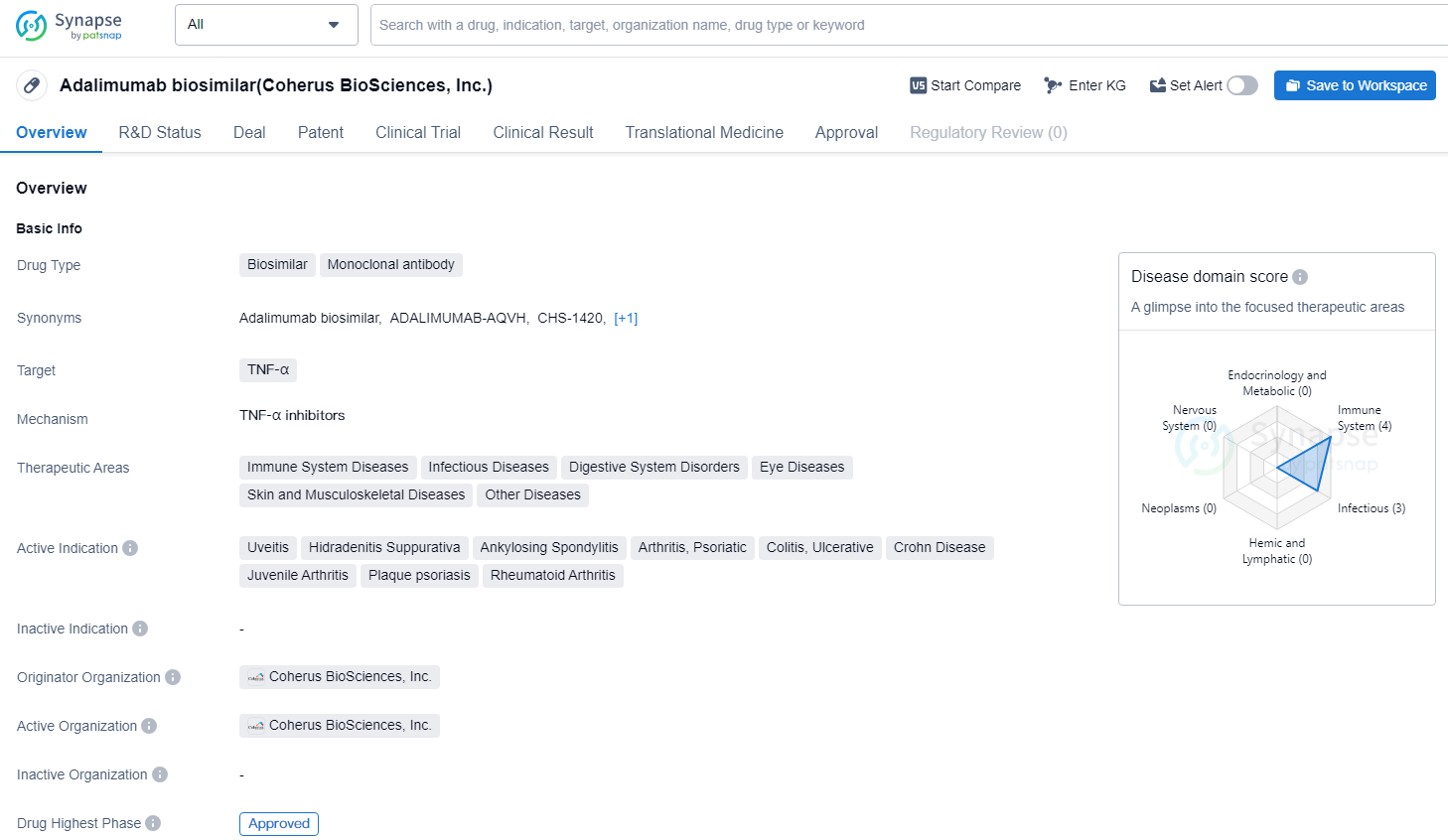
Adalimumab biosimilar is a monoclonal antibody biosimilar drug that targets TNF-α. It is approved for the treatment of a wide range of therapeutic areas, including immune system diseases, infectious diseases, digestive system disorders, eye diseases, skin and musculoskeletal diseases, and other diseases. The approval of Adalimumab biosimilar represents a significant milestone in the pharmaceutical industry, as it expands the options available for patients suffering from a variety of conditions.