Biocon Biologics Obtains Entry Timeline for US with its Stelara® Analog, Bmab 1200
Biocon Biologics Ltd, a wholly owned subsidiary of Biocon Ltd that operates globally in the biosimilars industry, declared today the signing of a resolution and licensing deal with Janssen Biotech Inc. and Johnson & Johnson. This agreement facilitates the path for the business to market Bmab 1200 in the U.S., a biosimilar candidate for Stelara®.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Under the terms of a newly formalized contract, the entity in question is authorized to introduce their product on the American market starting in February 2025, subject to endorsement from the U.S. Food and Drug Administration (FDA). The regulatory agency has acknowledged the entity's Submission for a Biologics License for the therapeutic agent Bmab 1200 (bUstekinumab) and is examining it via the 351 process.
Biocon Biologics alongside Janssen have come to a conclusive agreement, effectively resolving the ongoing litigation tied to the Inter Partes Review in connection with patent number US 10961307, which was filed before the Patent Trial and Appeal Board at the United States Patent and Trademark Office.
The Chief Executive Officer and Managing Director of Biocon Biologics Ltd, Shreehas Tambe, remarked, "Our alignment with this settlement demonstrates our dedication to scientific progress and inventive efforts. With bUstekinumab, Bmab 1200, we are gratified to position Biocon Biologics among the pioneering entities to provision a robust, superior biosimilar for medical professionals and individuals in need across the U.S."
Commenting on the significance of the understanding, the company’s Senior Officer for Commercial Strategy in Advanced Markets, Matthew Erick, stated, "The successful negotiation concerning our biosimilar of Ustekinumab signifies a critical juncture for our enterprise. It manifests our unwavering commitment to offering life-altering biosimilar medications at accessible rates. It highlights our tenacity in aiding those affected by inflammatory conditions and promoting significant improvements in the medical field."
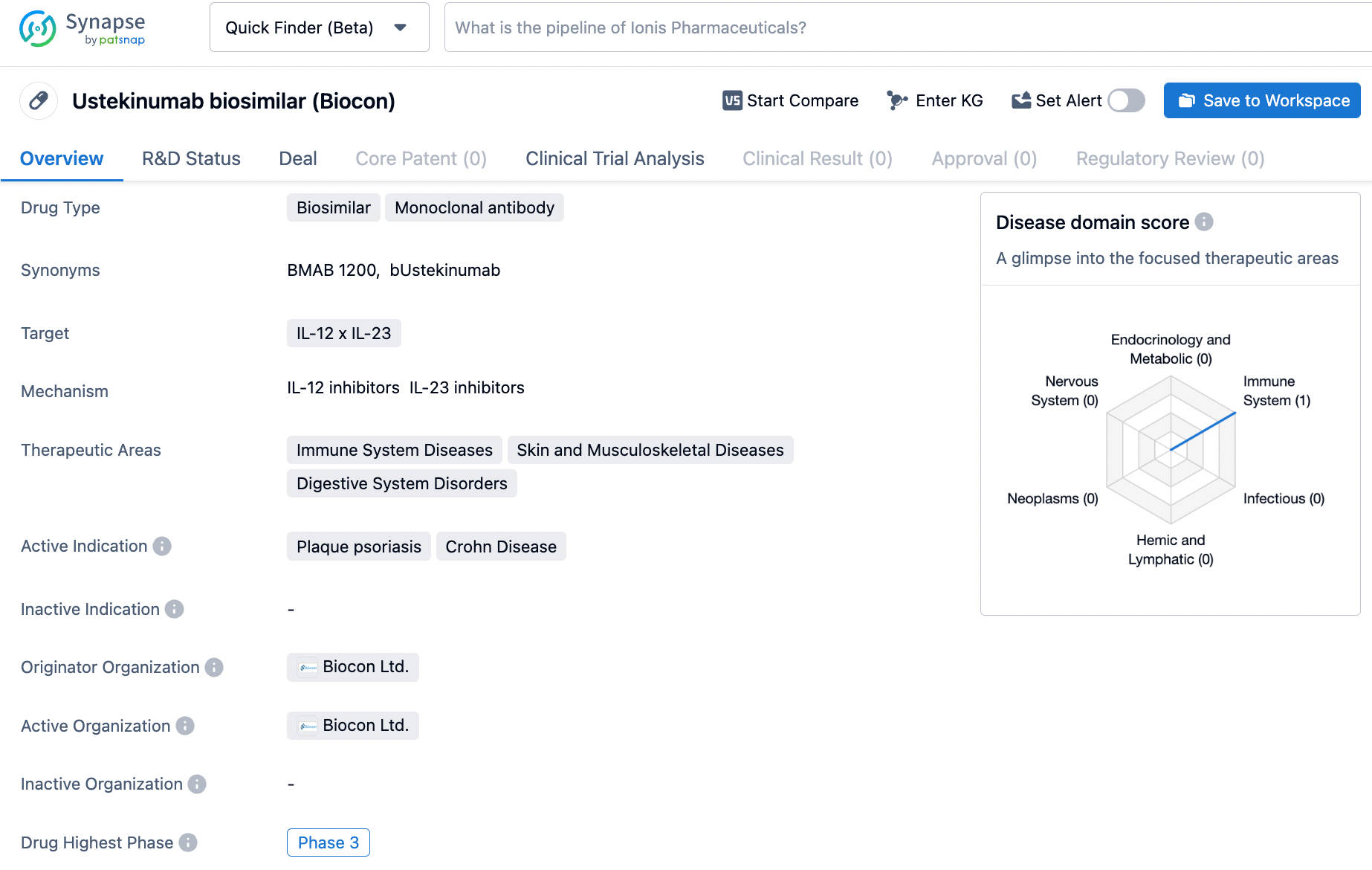
Stelara® (Ustekinumab) acts as a monoclonal antibody drug that blocks the improper modulation of the immune system via IL-12/23, and it has been sanctioned for use in disorders such as psoriasis, Crohn's disease, ulcerative colitis, plaque psoriasis, and psoriatic arthritis. In 2023, the reference brand Stelara® achieved a revenue pinnacle of $7 billion within the U.S. market.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
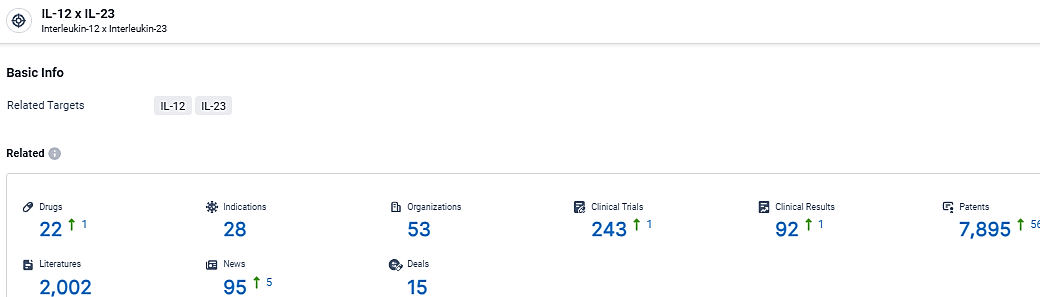
According to the data provided by the Synapse Database, As of March 4, 2024, there are 22 investigational drugs for the IL-12/23 target, including 28 indications,53 R&D institutions involved, with related clinical trials reaching 243, and as many as 7895 patents.
Ustekinumab biosimilar targets the IL-12 x IL-23 pathway. It shows potential in the treatment of immune system diseases, skin and musculoskeletal diseases, and digestive system disorders. With its highest phase of development being Phase 3, Ustekinumab biosimilar holds promise for patients suffering from plaque psoriasis and Crohn's disease, offering a potential alternative for effective and affordable treatment.