Ironwood Pharma Reports Positive Phase III Results for Apraglutide in Adult Short Bowel Syndrome
Ironwood Pharmaceuticals, Inc., a corporation specializing in gastrointestinal health, has disclosed encouraging primary outcomes from its critical STARS Phase III study. This research was centered on assessing the performance and security of a weekly subcutaneous injection of apraglutide for diminishing the reliance on parenteral nutrition among adults suffering from short bowel syndrome accompanied by intestinal failure.
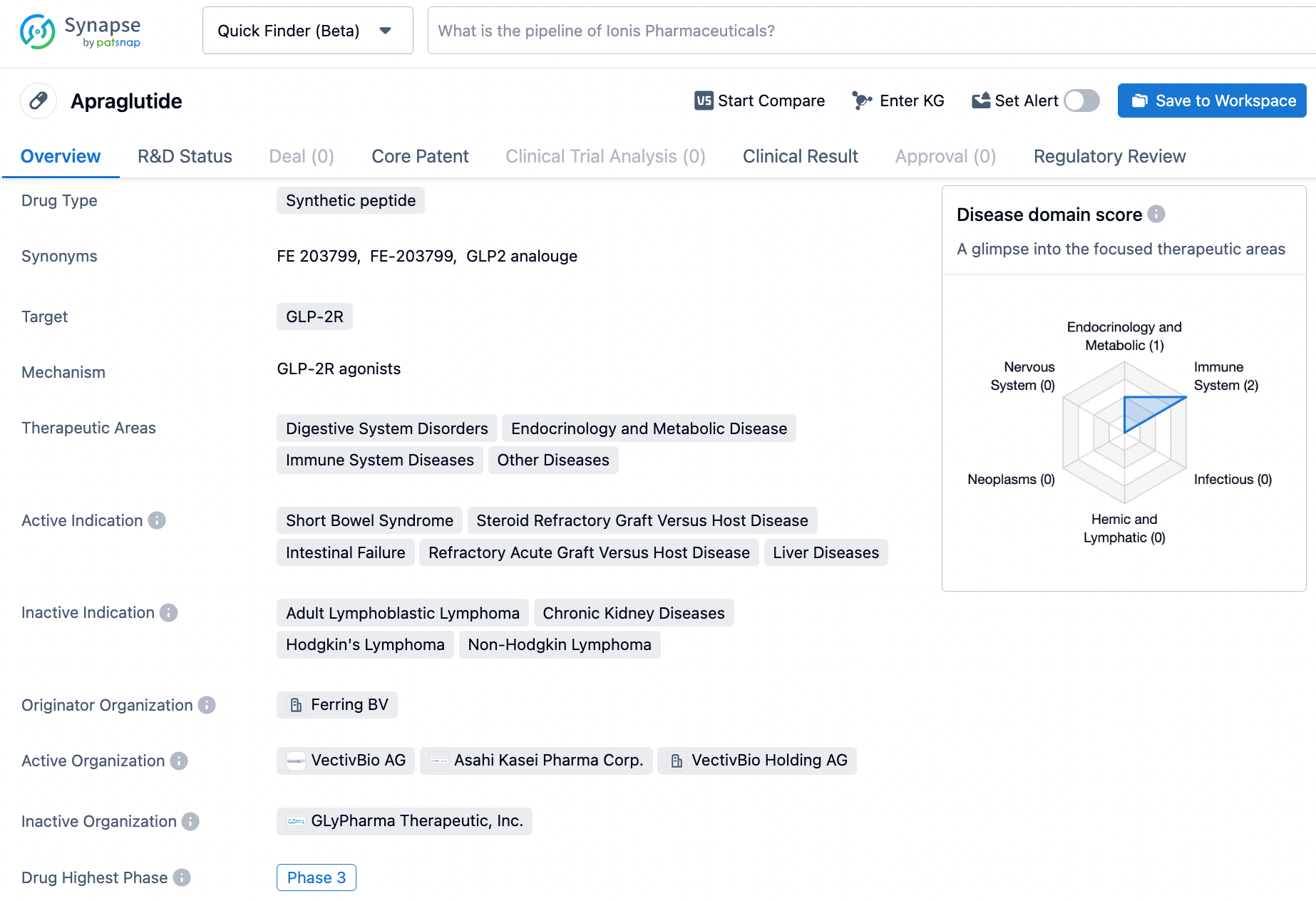
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
SBS-IF is an infrequent yet serious condition of organ failure, where around 18,000 adults in the US, Europe, and Japan rely on parenteral support (PS). With this background, Ironwood is preparing to seek approval for the drug apraglutide by filing a new drug application for its use in adults with SBS who rely on PS.
The international trial, carried out across multiple centers, was blinded, randomized, and controlled by placebo to assess apraglutide's effectiveness and safety, administered through weekly subcutaneous injections for adult SBS-IF patients. The study achieved its primary target by demonstrating a significant reduction relative to the baseline in the weekly volume of PS at the 24-week mark when apraglutide was compared with the placebo.
Kishore R Iyer, an expert in Intestine Rehabilitation & Transplantation at The Mount Sinai Hospital, emphasized the importance of minimizing the need for parenteral support and lessening the therapeutic burdens for SBS-IF patients. He noted the STARS trial's top-line significance, highlighting it as the inaugural positive Phase III trial of a placebo-controlled nature in SBS-IF patients treated with a once-a-week GLP-2 analog.
Michael Shetzline pointed out that SBS-IF patients face a profound affliction paired with a demanding treatment protocol that requires extensive hours dedicated to parenteral support, affecting their life quality and presenting a risk of critical complications, including infections. He expressed optimism that the trial outcomes suggested apraglutide's potential to refine the current treatment standard for SBS adults dependent on parenteral support, positioning it uniquely as the sole GLP-2 analog that is administered weekly, pending approval. Shetzline extended gratitude to those who participated in the most extensive GLP-2 analog study within the SBS-IF community and committed to collaborating with regulatory bodies to aim for apraglutide's availability for individuals affected by this challenging condition.
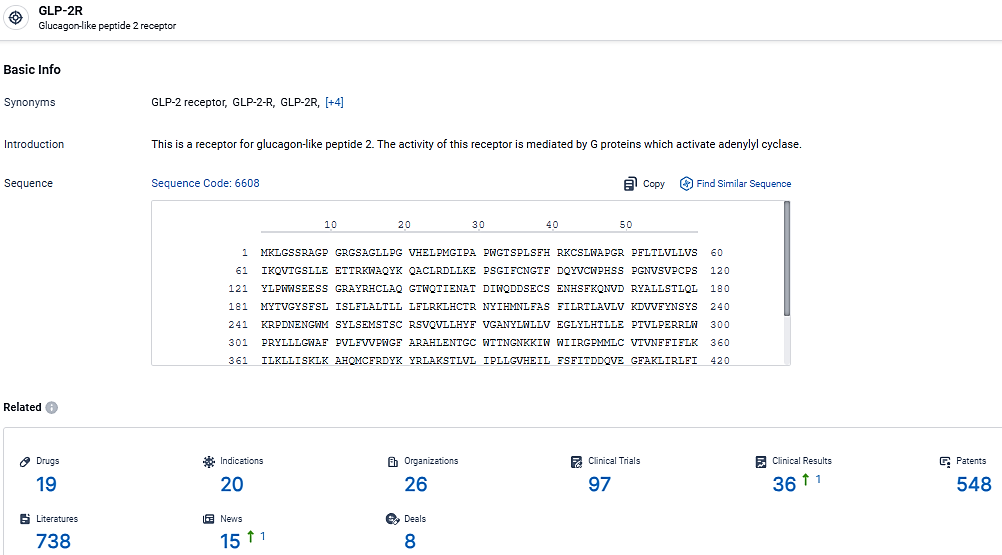
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 4, 2024, there are 19 investigational drugs for the GLP-2R target, including 20 indications,26 R&D institutions involved, with related clinical trials reaching 97, and as many as 548 patents.
Apraglutide is an investigational, next-generation, long-acting synthetic GLP-2 analog being developed for a range of rare gastrointestinal diseases where GLP-2 can play a central role in addressing disease pathophysiology, including short bowel syndrome and Acute Graft-Versus-Host Disease.