BioRay begins Phase I trial for BRY812, a novel LIV-1 antibody drug conjugate, with its first patient
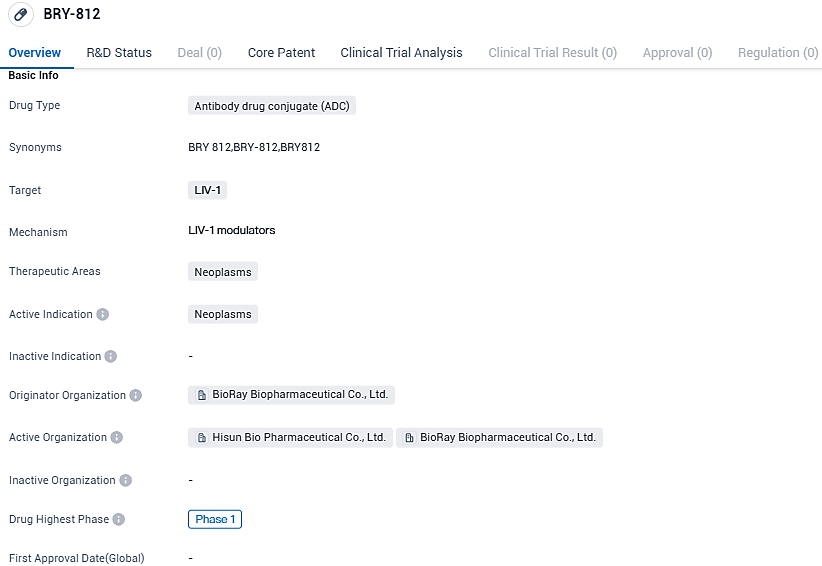
BioRay Pharmaceutical Co., Ltd. communicated news of having administered the initial dosage to a patient in the Phase I Clinical trial of BRY812. This drug is a third-generation antibody-drug conjugate engineered to target LIV-1 for treating advanced harmful tumours. Leading the clinical trial is Sun Yat-sen Memorial Hospital which is part of Sun Yat-sen University, with primary investigators being academician Song Erwei and Professor Yao Herui.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
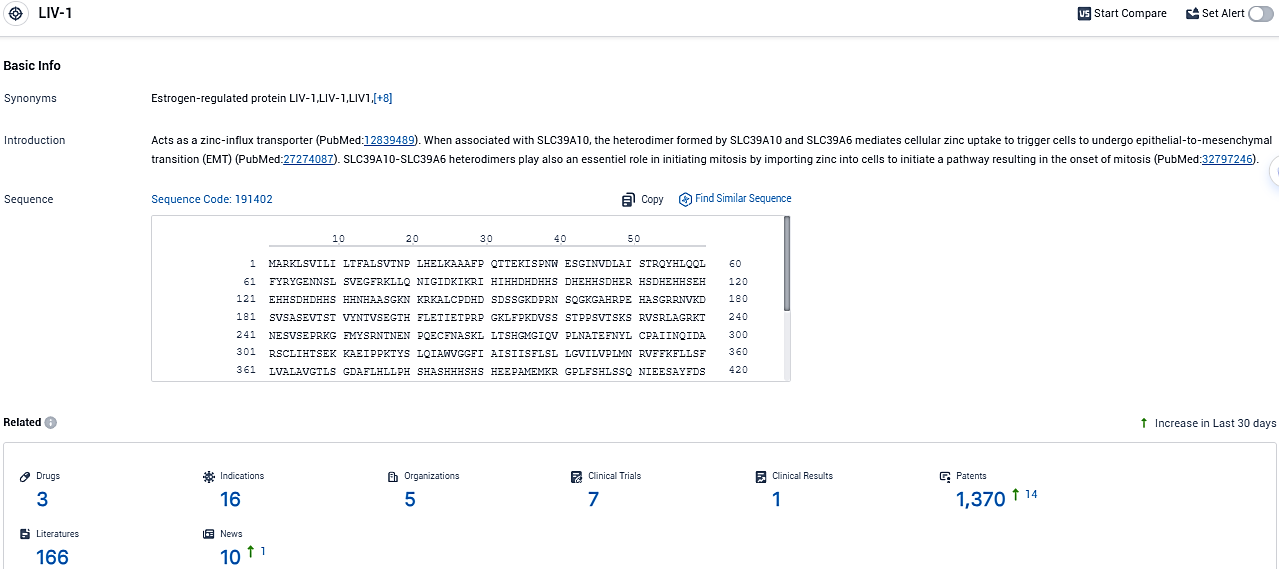
Recognized as SLC39A6, ZIP6, or LIV-1, it's a multipass transmembrane protein that's part of the ZIP family of zinc transporters, exhibiting zinc transporter and metalloproteinase features. LIV-1 is actively involved in maintaining the balance of intracellular zinc ions metabolism, and aids in moving these ions from external spaces or internal organelles to the cytoplasm, affecting cell growth.
Hitherto, there are no globally acknowledged drugs designed to take on LIV-1, which makes BioRay's BRY812 the premier LIV-1 ADC in China and the second one to proceed to international clinical trials. BRY812 employs the proprietary CysLink™ irreversible chemical conjugation technology and a robust linker for the antibody-toxin conjugation.
In preliminary studies, BRY812 demonstrated a significant reduction in tumor growth and exhibited exceptional anti-tumor efficacy, potentially outdoing similar medications. When compared to other similar class drugs, it displays a high degree of stability in circulation, ensures the effective delivery of the payload within the tumor while drastically cutting back on toxin shedding and serum exchange. This grants BRY812 a favorable safety score and an enhanced therapeutic window.
Dr. Zhu Wei, BioRay's CMO, articulated, "The ADCs' significant market potential necessitates market competition differentiation and patient population coverage expansion. As the pioneering domestic ADC that focuses on LIV-1, and has advanced to clinical trials, BRY812 is expected to be beneficial in treating several advanced malignant tumors, fulfilling more clinical medication requirements, and potentially allowing for more therapy choices for patients."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 16, 2023, there are 3 investigational drugs for the LIV-1 target, including 16 indications, 5 R&D institutions involved, with related clinical trials reaching 7, and as many as 166 patents.
BRY-812 targets LIV-1 and is intended for the treatment of neoplasms. The drug is currently in Phase 1 of clinical development, suggesting that it is still in the early stages of testing. Further research and clinical trials will be needed to determine the safety, efficacy, and potential therapeutic applications of BRY-812 in the treatment of neoplasms.