Ventyx Biosciences announces Phase 2 results for VTX958 in psoriasis and provides a company update
Ventyx Biosciences, Inc., a medical research firm in the clinical phase, which is dedicated to developing new oral treatments to tackle various unmanaged inflammatory ailments, shared its Phase 2 clinical trial findings for its compound VTX958 on patients affected with moderate to serious plaque psoriasis and also provided a business progress update.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
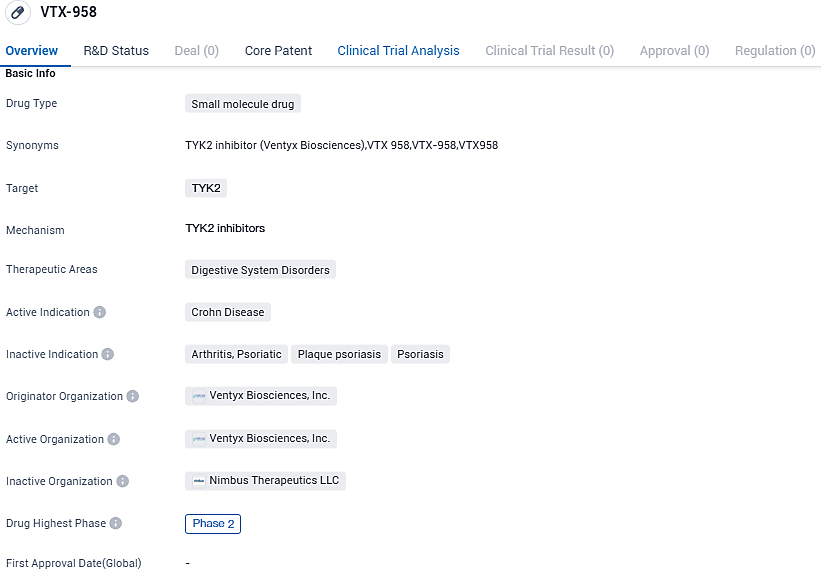
"Even though the Phase 2 trial of VTX958 in plaque psoriasis satisfied its primary and significant secondary outcomes, the actual degree of effectiveness left us wanting, despite the drug's target exposure level being achieved in the trial.” stated the founder and CEO, Dr. Raju Mohan. “In light of these findings, no further progress of VTX958 in the intensely competitive markets of psoriasis and psoriatic arthritis can be approved.
I would like to extend my gratitude to the patients, investigators involved, as well as acknowledge the Ventyx team's commitment to carrying out these trials," added Mohan. The SERENITY trial, a Phase 2 research, was a four-month long, randomized, double-blind, placebo-controlled, dosage varied analysis of VTX958 effectiveness and safety in dealing with moderate to severe plaque psoriasis cases.
The primary goal was seeing a 75% decrease in the Psoriasis Area and Severity Index by the end of this duration. At week 16, high doses of VTX958 had statistically relevant outcomes in both primary and key secondary endpoints. No severe drug-related adverse events were noted. Although the trial satisfied its primary objective, the effectiveness level noted didn't quite hit the mark as per our internal goal, thereby disrupting the further progress of VTX958 in plaque psoriasis trials.
As a result, we are promptly bringing to a halt all active processes tied to the Phase 2 trial for plaque psoriasis. Consequently, we have also decided to end the current Phase 2 testing of VTX958 for psoriatic arthritis.
However, the enrollment for the present Phase 2 trial for VTX958 in Crohn’s disease will persist, as we make plans for an interim effectiveness analysis to be done in early 2024.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
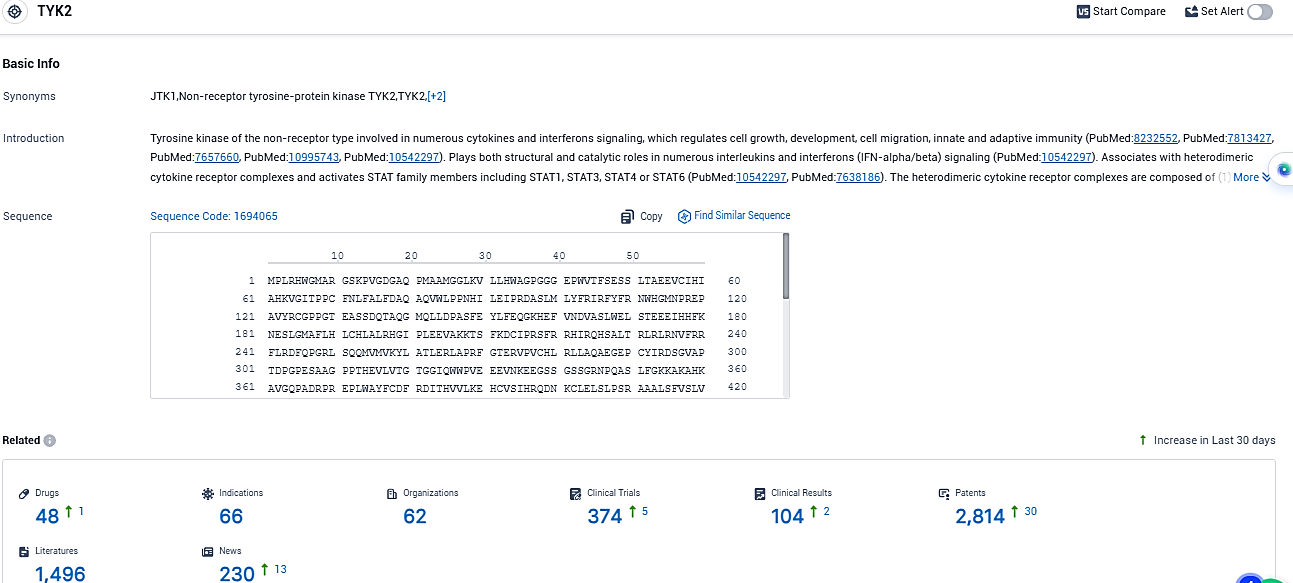
According to the data provided by the Synapse Database, As of November 16, 2023, there are 48 investigational drugs for the TYK2 target, including 66 indications, 62 R&D institutions involved, with related clinical trials reaching 374, and as many as 2814 patents.
VTX-958 is being developed for the treatment of Crohn's Disease, a digestive system disorder. Currently in Phase 2, VTX-958 shows potential as a therapeutic option for patients suffering from this chronic inflammatory condition.