FDA Approves CDR-Life's Clinical Trial for Solid Tumor Drug CDR404
CDR-Life Inc. has declared that the U.S. Food and Drug Administration has approved their request to begin studies on a new experimental therapy. The investigational drug, known as CDR404, is the company’s primary project aimed at creating targeted immunotherapy treatments for various solid cancerous growths.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
CDR404 represents a pioneering therapeutic agent, integrating an antibody-derived, dual-function, and bispecific molecule that is designed to engage T-cells targeting MAGE-A4 through the interaction with the major histocompatibility complex, using the proprietary M-gager platform from this biotech firm.
"With CDR404, we aim to deliver a readily available treatment solution for a variety of malignancies which express MAGE-A4 and currently lack effective therapies, such as non-small cell lung cancer," commented Dr. Christian Leisner, the CEO of CDR-Life.
Dr. Leisner expressed excitement about reaching this significant progress point and emphasized the company's ongoing efforts to develop a pipeline of additional candidates that utilize the M-gager technology. These are intended to address key intracellular targets in cancer, with the objective of enhancing the quality of patients' health outcomes.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
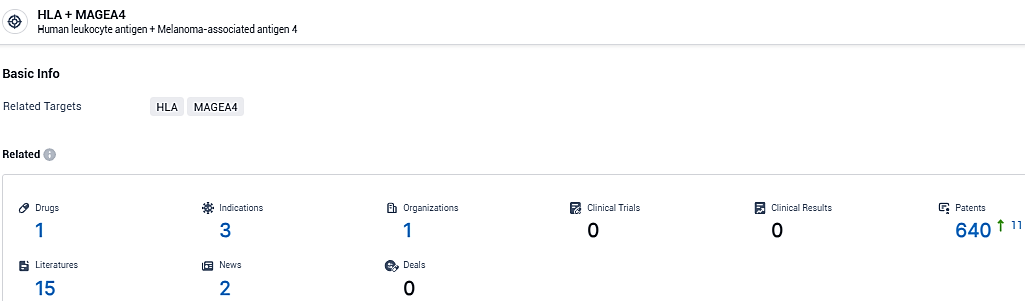
According to the data provided by the Synapse Database, As of January 29, 2024, there are 1 investigational drugs for the HLA and MAGEA4 target, including 3 indications, 1 R&D institutions involved, and as many as 640 patents.
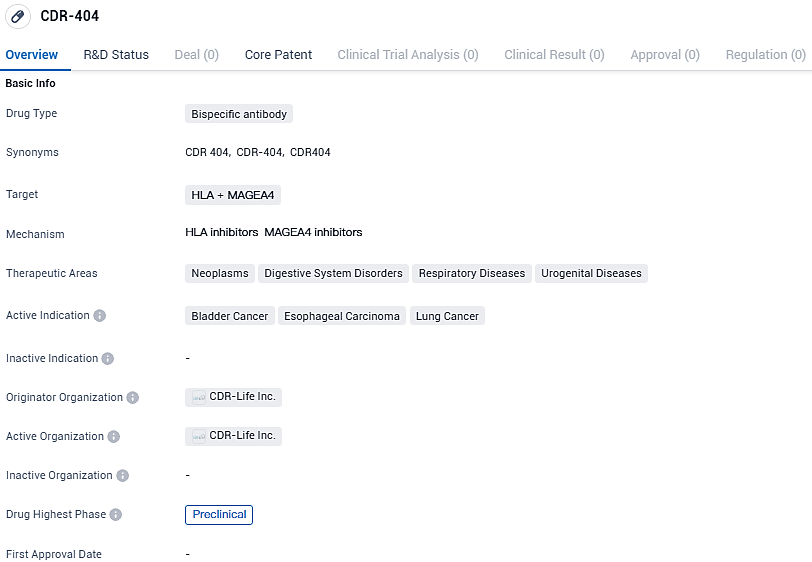
CDR-404 targets HLA and MAGEA4 and has potential applications in neoplasms, digestive system disorders, respiratory diseases, and urogenital diseases. The drug is currently in the preclinical phase and shows promise as a potential treatment for bladder cancer, esophageal carcinoma, and lung cancer. CDR-Life Inc. is the originator organization behind CDR-404 and is dedicated to advancing innovative therapies in the pharmaceutical industry.