Blocking Cellular Protein CypA Thwarts Poxviruses' Sneaky Tactics, Offering Potential Monkeypox Treatment
Scientists from the University of Oxford, University of Cambridge, and The Pirbright Institute in the UK have discovered a potential new treatment approach for poxviruses like monkeypox and smallpox viruses. They were investigating how poxviruses evade the natural defense mechanisms of human cells. Previously, they have found that poxviruses hijack a specific cellular protein, cyclophilin A (CypA), to avoid host cell defenses, enabling effective replication and spread.
The hijacking mechanism
In fact, existing immunosuppressive drugs and antivirals all target this same cellular protein CypA. In their new study, the researchers found that these drugs can also restrict poxvirus replication and spread. With this approach, the drugs do not directly target the viruses, meaning the viruses will have difficulty evolving resistance. Since this hijacking mechanism is conserved across poxviruses, the drugs could effectively treat various diseases caused by poxviruses like monkeypox virus and variola virus, which causes smallpox.
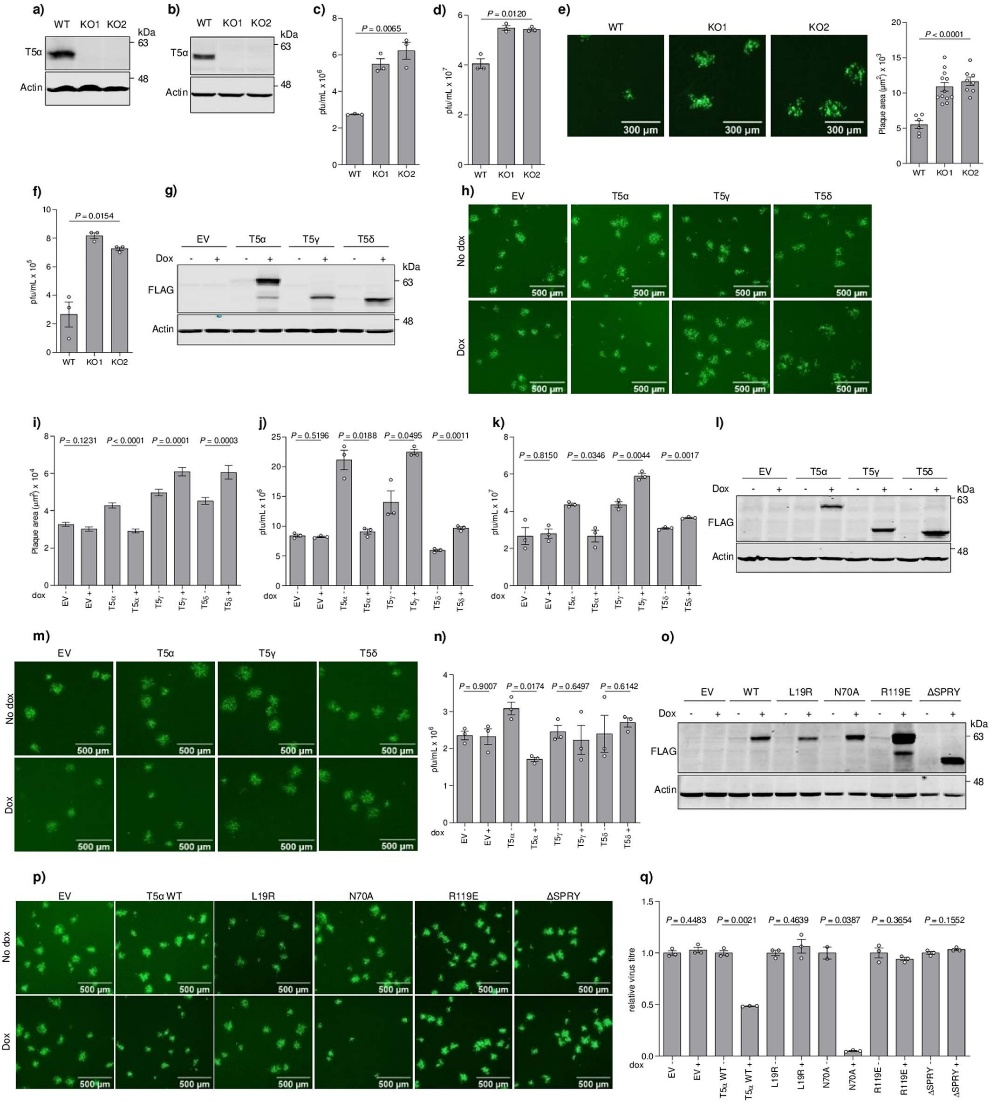
The project commenced with the observation that vaccinia virus (VACV) infection leads to a decrease in TRIM5α levels in human cells. By utilizing engineered cells lacking TRIM5α, the research team discovered an enhancement in viral replication and spread, suggesting that TRIM5α possesses antiviral activity.
Subsequently, the researchers identified the specific VACV proteins that are targeted by TRIM5α. They also uncovered that the virus employs CypA to hinder TRIM5α's antiviral activity, and it produces the C6 protein to induce the destruction of TRIM5α.
Given that existing drugs are designed to target CypA, the researchers conducted tests using these drugs against poxviruses, including the monkeypox virus. The results demonstrated that the drugs exhibited antiviral effects by rendering the viruses more susceptible to TRIM5α.
Intricate interactions
The experiments conducted in this study provide valuable insights into the intricate interaction between TRIM5α, viral proteins, and cellular factors.
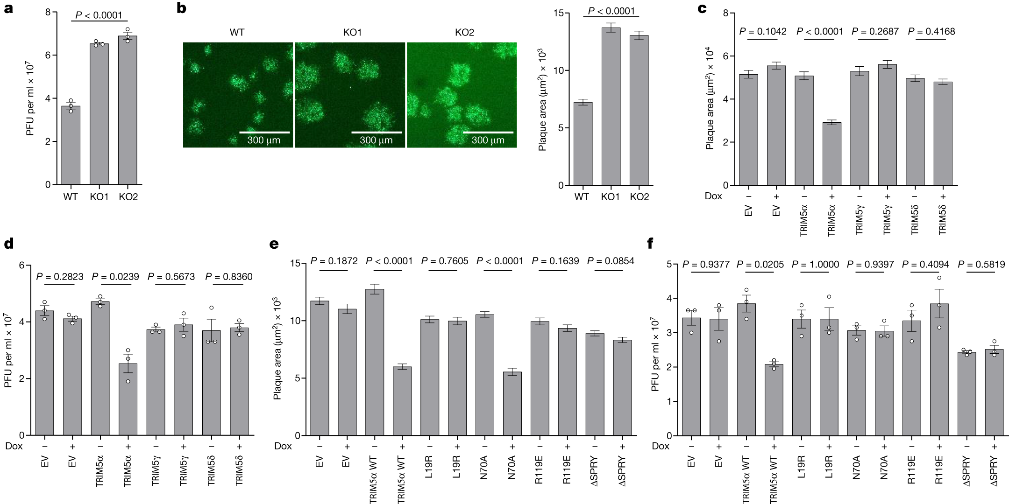
First and foremost, the researchers provide evidence that TRIM5α, previously known for its role in restricting certain RNA viruses like HIV, also possesses the ability to restrict orthopoxviruses, a group of DNA viruses. They reveal that TRIM5α achieves this by binding to the capsid protein L3 of orthopoxviruses through its SPRY domain. This interaction leads to the suppression of virus replication and activation of innate immunity.
To overcome the restriction imposed by TRIM5α, orthopoxviruses employ two distinct strategies. The first strategy involves a viral protein called C6, which interacts with TRIM5α through its RING domain. This interaction triggers the degradation of TRIM5α via the proteasome pathway. The researchers validate this degradation phenomenon by utilizing VACV mutants lacking specific gene blocks. Notably, the mutant v6/2, lacking the C6 gene, fails to induce TRIM5α degradation.
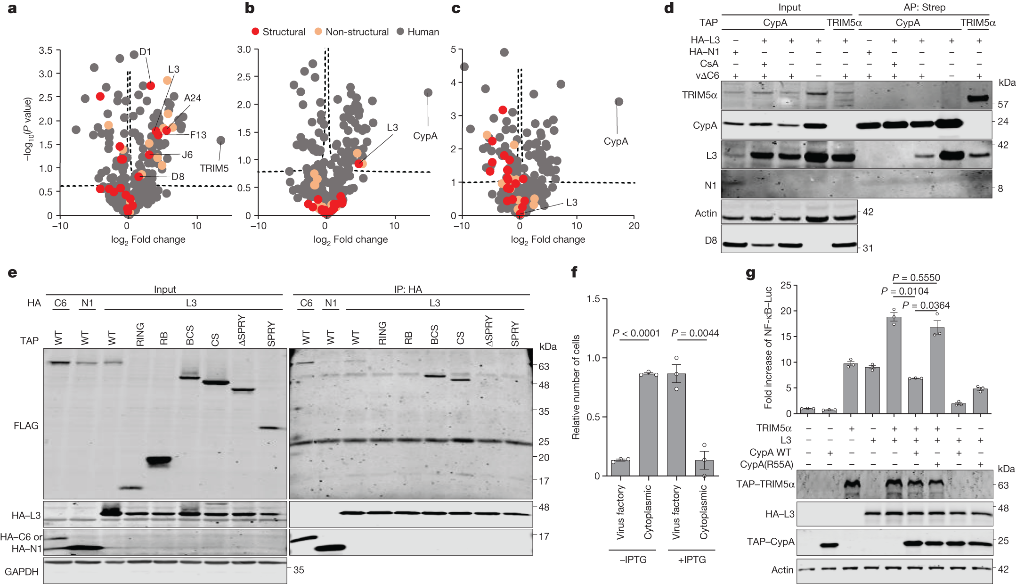
The study further delves into the intricate mechanism underlying C6-mediated TRIM5α degradation. The researchers discover that C6, possessing a predicted BCL-2-like fold, co-precipitates with TRIM5α during VACV infection. Through experiments utilizing TRIM5α mutants lacking autoubiquitylation activity, they hypothesize that C6 likely co-opts cellular E3 ligases to ubiquitylate TRIM5α, resulting in its subsequent proteasomal degradation.
The second countermeasure employed by orthopoxviruses involves the recruitment of CypA through its interaction with the capsid protein L3. CypA acts as an antagonist to TRIM5α, inhibiting its antiviral activity. The interaction between CypA and TRIM5α is effectively prevented by cyclosporine A (CsA) and its derivatives, such as alisporivirand NIM811, which specifically target CypA. Importantly, the proviral effect of CypA and the antiviral effect of CsA are demonstrated to be dependent on TRIM5α.
Additionally, the researchers investigate the potential antiviral activity of CsA and its derivatives against orthopoxviruses. They find that these drugs, which target CypA, exhibit significant antiviral activity against orthopoxviruses. Importantly, due to their targeting of a host factor, the emergence of viral drug resistance to CsA and its derivatives is expected to be challenging. Consequently, the study suggests evaluating CsA derivatives as potential antiviral agents against orthopoxviruses, including monkeypox and variola virus (the causative agent of smallpox).
The lingering specter
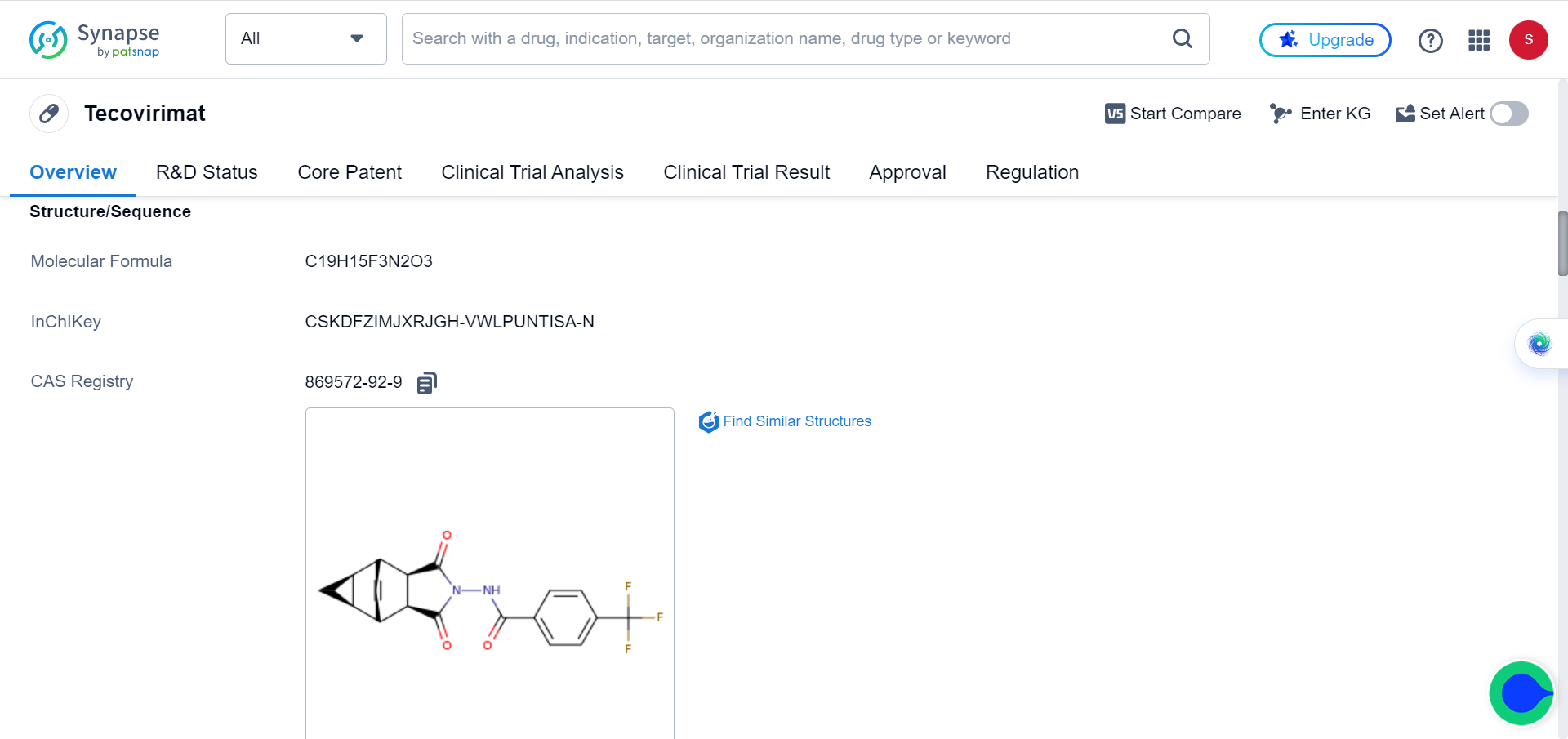
Although smallpox was eradicated in 1979, variola virus is maintained in two highly secure laboratories, one in the US and one in Russia, as it could potentially be used as a biological weapon. Tecovirimat is approved to treat smallpox.
Despite a decline in UK cases, the Monkeypox virus continues to circulate. However, the use of tecovirimat against severe monkeypox last year resulted in the emergence of resistant strains, highlighting the need for alternative treatments. Co-corresponding author of the study, Professor Geoffrey L. Smith expressed optimism, stating that the newly discovered drugs could exhibit greater durability against monkeypox and other poxviruses, including smallpox.
Poxviruses encounter cellular proteins that impede their replication and spread within host cells. The researchers identified two key proteins: TRIM5α, which restricts viral growth, and CypA, which inhibits TRIM5α. By targeting CypA, existing drugs can sensitize poxviruses to the inhibitory effects of TRIM5α. Professor Smith emphasized the advantage of repurposing drugs that already target CypA, as they have undergone clinical trials, enabling a faster drug development process.
Furthermore, poxviruses pose a threat to animals, such as the ongoing global outbreak of 'lumpy skin disease' among cattle. Professor Smith highlighted the unexpected nature of their findings, explaining that their initial research into poxvirus evasion of host defenses unexpectedly led to the discovery of potential drugs against monkeypox and other poxviruses.
The establishment of the UK monkeypox research consortium played a crucial role in facilitating a swift and coordinated scientific response by public funders and policymakers. Professor Guy Poppy, the Interim Executive Chair of the Biotechnology and Biological Sciences Research Council, emphasized the urgency of such collaborative efforts in addressing emerging infectious diseases like monkeypox.
Reference
Zhao, Y., Lu, Y., Richardson, S. ,Smith G.L., et al. TRIM5α restricts poxviruses and is antagonized by CypA and the viral protein C6. Nature (2023). https://doi.org/10.1038/s41586-023-06401-0.