Cytokine Engineering: Tailoring Immune Messengers for Therapeutic Applications
Cytokines are small secreted proteins that play a key role in many cellular functions such as development, differentiation, growth and survival. Given their wide-ranging effects throughout the body, precisely regulating cytokine activity is crucial from both a physiological and pathological standpoint. As a result, scientists have focused significant research efforts on engineering cytokines and their receptors to safely and effectively modulate cytokine signaling for therapeutic benefit.
Interleukin-2 (IL-2) in particular has undergone extensive engineering attempts to generate IL-2 variants with selective activities. Some variants preferentially stimulate regulatory T cells, showing promise for treating autoimmune diseases, while others preferentially activate effector T cells, pointing to potential applications for cancer immunotherapy.
Engineering is also being applied to other cytokines like IL-10, interferons and IL-1 family members due to their immunosuppressive, antiviral and antitumor properties. Strategies to modulate cytokine actions encompass a wide range, including altering receptor binding affinities, prolonging cytokine half-lives in vivo, and fine-tuning downstream signaling outcomes. This field is rapidly expanding as intensive work aims to develop improved treatments across many disease areas by leveraging cytokine engineering approaches. The drug development strategies involved can be broadly divided into either enhancing or inhibiting the activities of cytokines.
Cytokines and Receptors
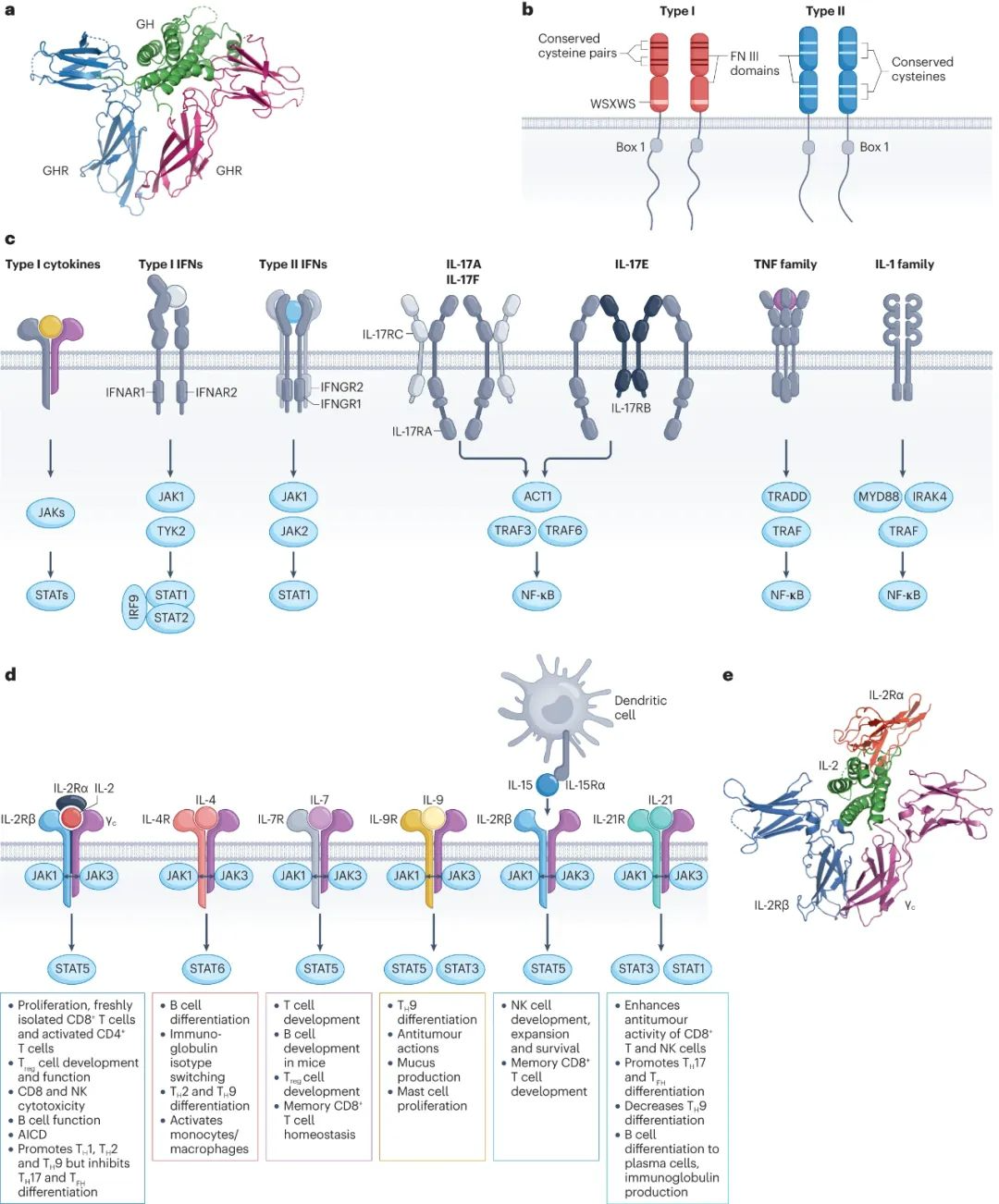
Cytokines control various cellular processes such as development, differentiation, growth, survival, and death, but they can also induce pathological effects, such as inflammation and autoimmune diseases. Cytokines encompass type I, type II, and other families (including IL-1, IL-17, TNF, and TGFβ). The review primarily explores type I and II cytokines, with a special focus on IL-2.
These cytokines primarily utilize the JAK-STAT signaling pathway. Type I cytokines typically bind to homodimeric or heterodimeric receptors, while other cytokines exhibit more diverse receptor structures. Two critical factors in cytokine therapy development are pleiotropy and redundancy. Pleiotropy represents the mediation of multiple effects, typically across various cell types, while redundancy refers to the ability of two or more cytokines to induce similar or identical effects.
Strategies to Enhance Cytokine Activity
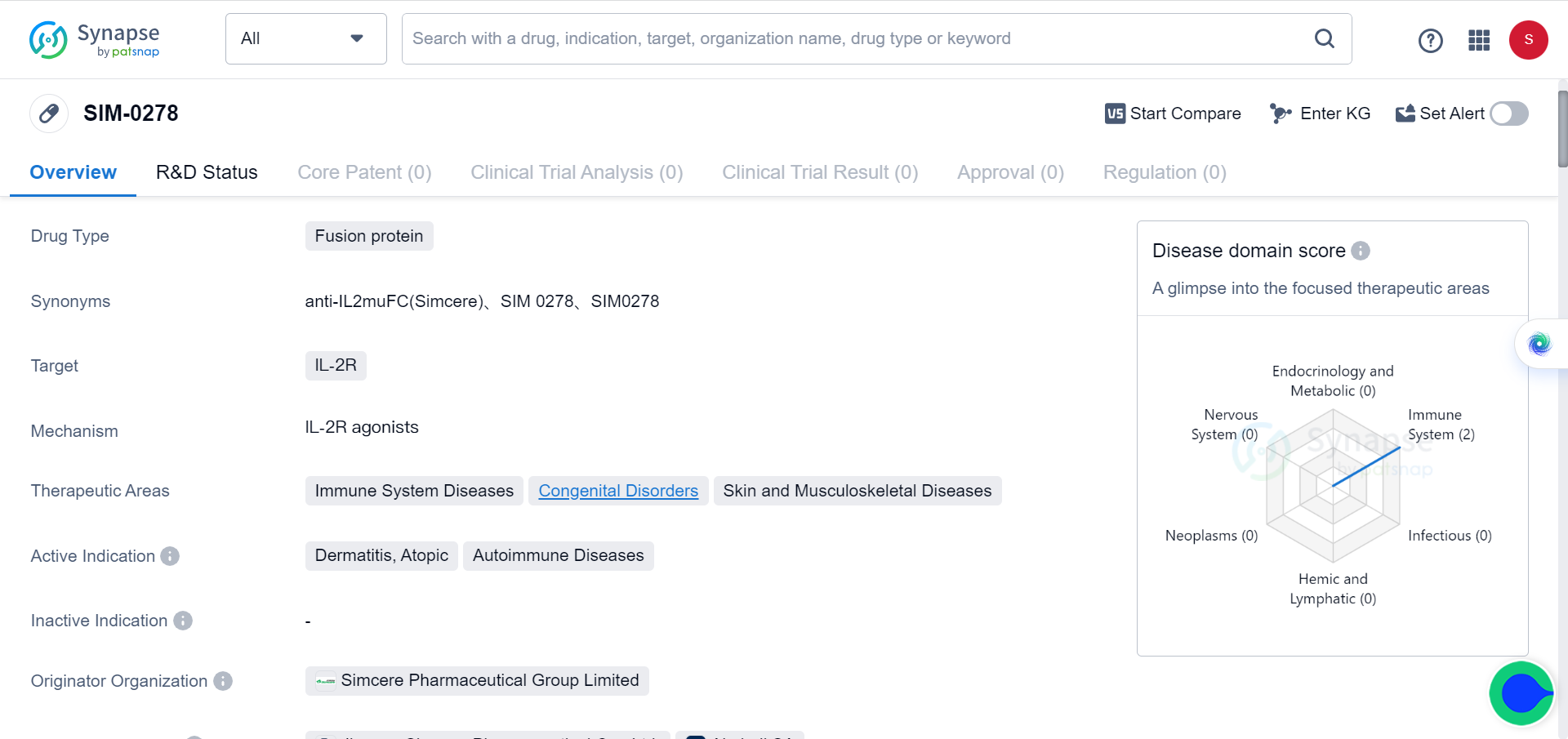
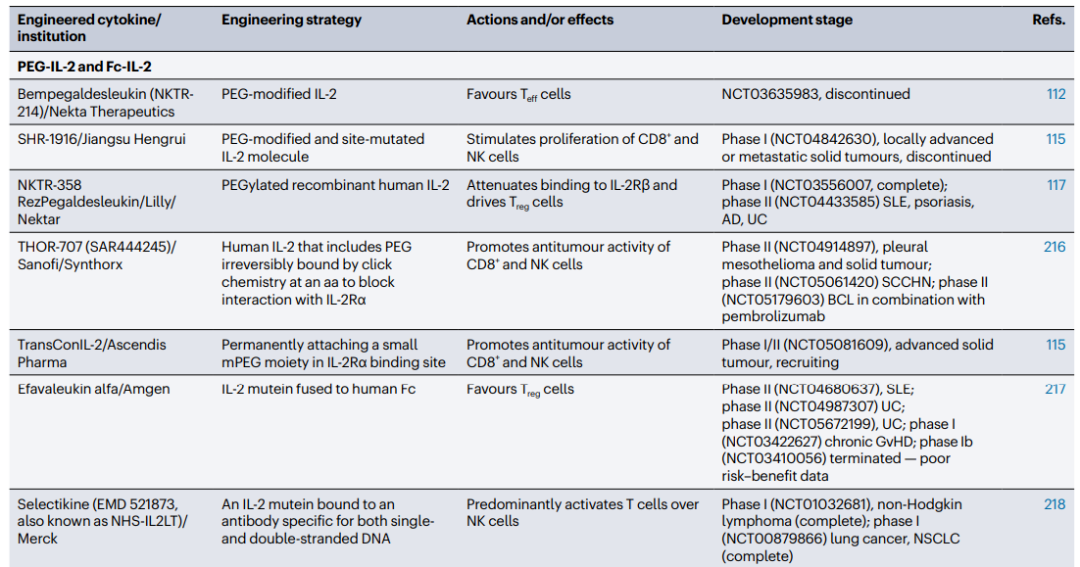
Extending cytokine half-life through protein fusion and PEGylation has proven to be an effective strategy. Administering native cytokines is often limited by their short in vivo half-life and potential toxicity. However, there are various methods available to extend their half-life. One approach is to fuse cytokines with proteins such as immunoglobulins, Fc regions, or albumin. For instance, Simcere's IL-2 mutant Fc fusion protein SIM0278, which was licensed overseas last year for a staggering $500 million, has shown promising results. Additionally, IL4-albumin fusion drugs have also demonstrated potential.
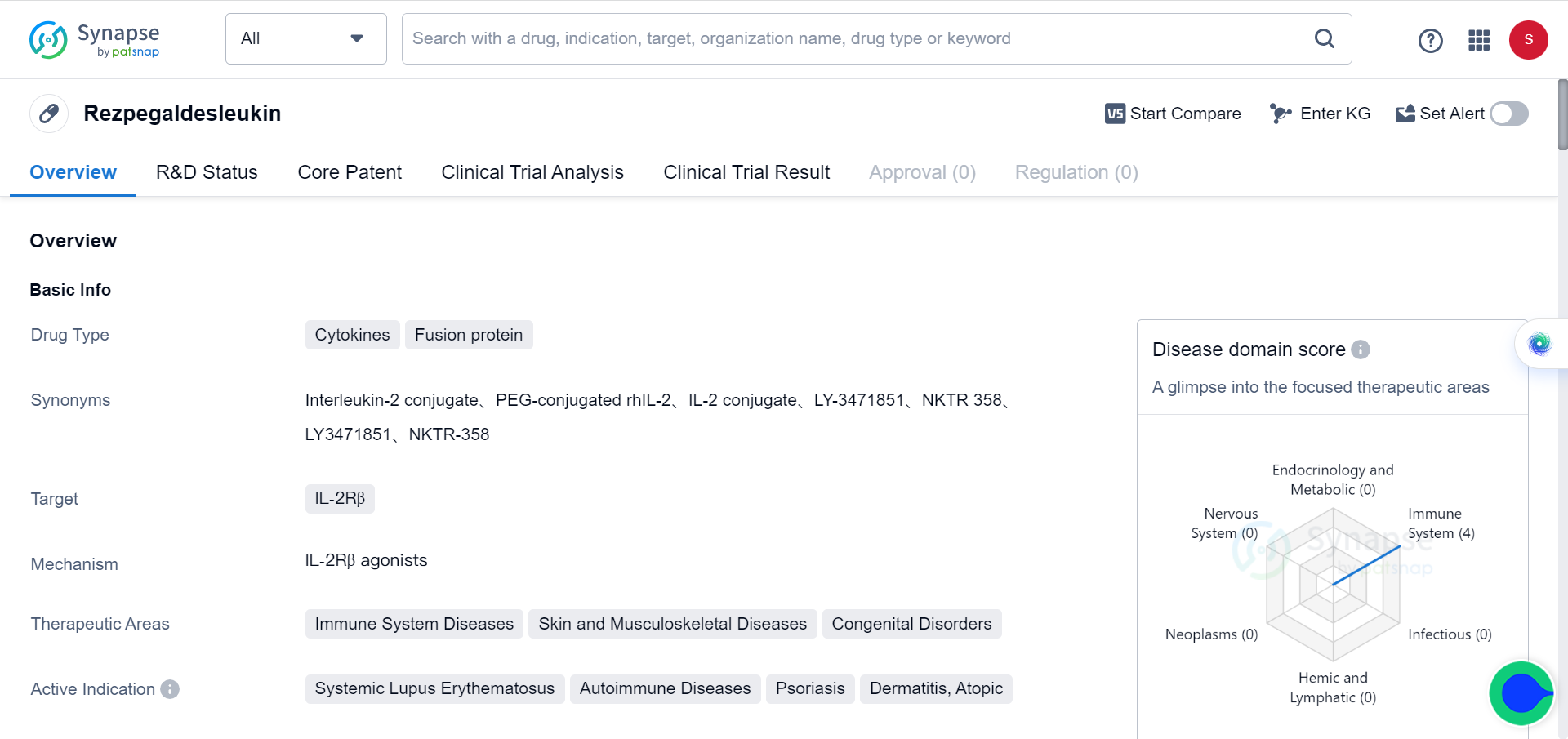
Another effective approach for extending protein half-life in vivo is PEGylation, a technique that has been used for decades. PEGylation helps prevent degradation and reduces immunogenicity. Notably, Nektar's PEGylated NKTR-358 has exhibited a longer half-life and significant efficacy in lupus mouse models.
Optimizing cytokine delivery
Various methods have been explored to facilitate cytokine delivery, including the use of polymer matrices implanted near tumors, microparticles (>1 μm) such as polymeric microparticles with lipophilic or solid polymers, and nanoparticles (10-100 nm) that possess unique properties due to their small size.
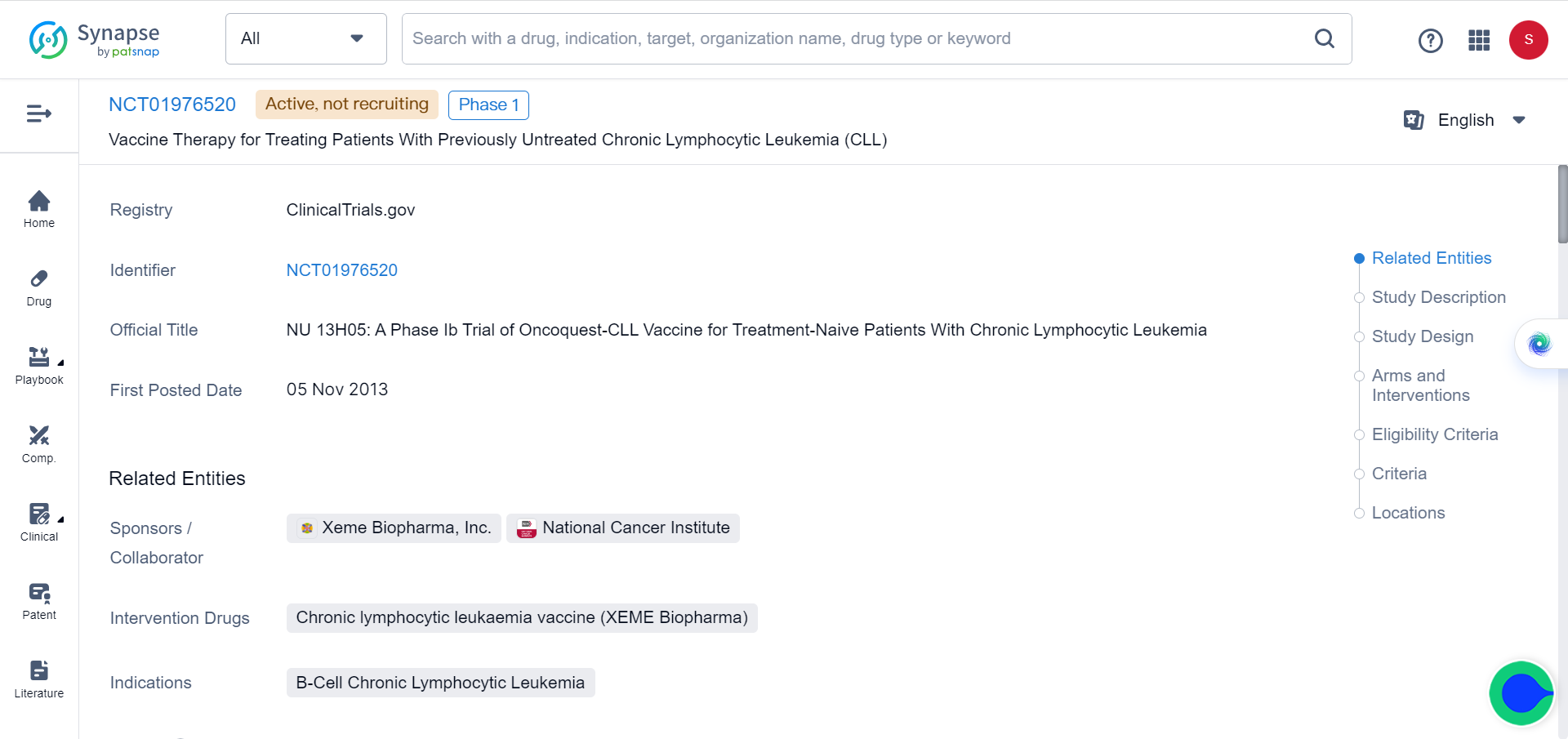
Although experience with these methods is limited, there have been promising results from clinical trials involving IL-2 delivery through large liposomal particles as part of cancer vaccines for lymphoma and leukemia treatment (NCT02194751and NCT01976520). Furthermore, selective activation of cytokines within the tumor microenvironment (TME) has also been investigated.
Studies have demonstrated that intratumoral injection of cytokine-aluminum hydroxide complexes can elicit potent and safer systemic anti-cancer immune responses. These findings highlight the potential of these approaches in cancer immunotherapy.
Immune complexes and immunocytokines
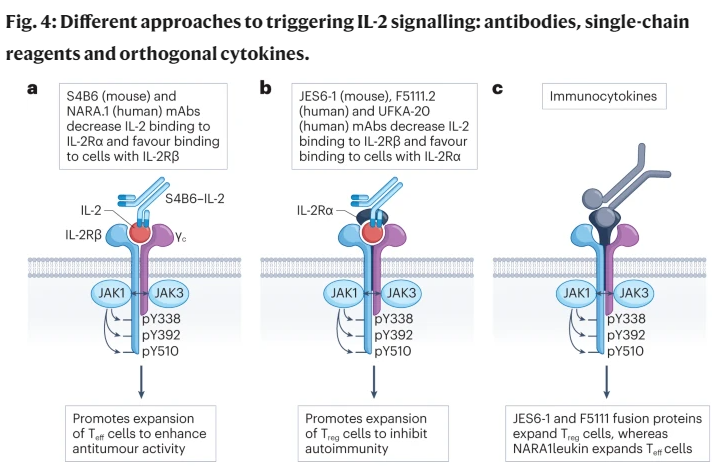
This encompasses various strategies, such as cytokine-anti-cytokine complexes or immune complexes, involving cytokine-antibody fusions, as well as cytokines fused to monoclonal antibodies (mAbs) or antibody fragments.
Another approach involves engineered cytokine receptors or cytokine-receptor pairs, which can be modified to enhance their functionality. Orthogonal cytokines offer an alternative approach by modifying the extracellular domains of both cytokines and receptors while maintaining the signaling cytoplasmic domains.
Similarly, another method involves retaining the extracellular domains of cytokines and receptors while altering the cytoplasmic domains to achieve desired changes in signaling.
Mutant cytokine proteins
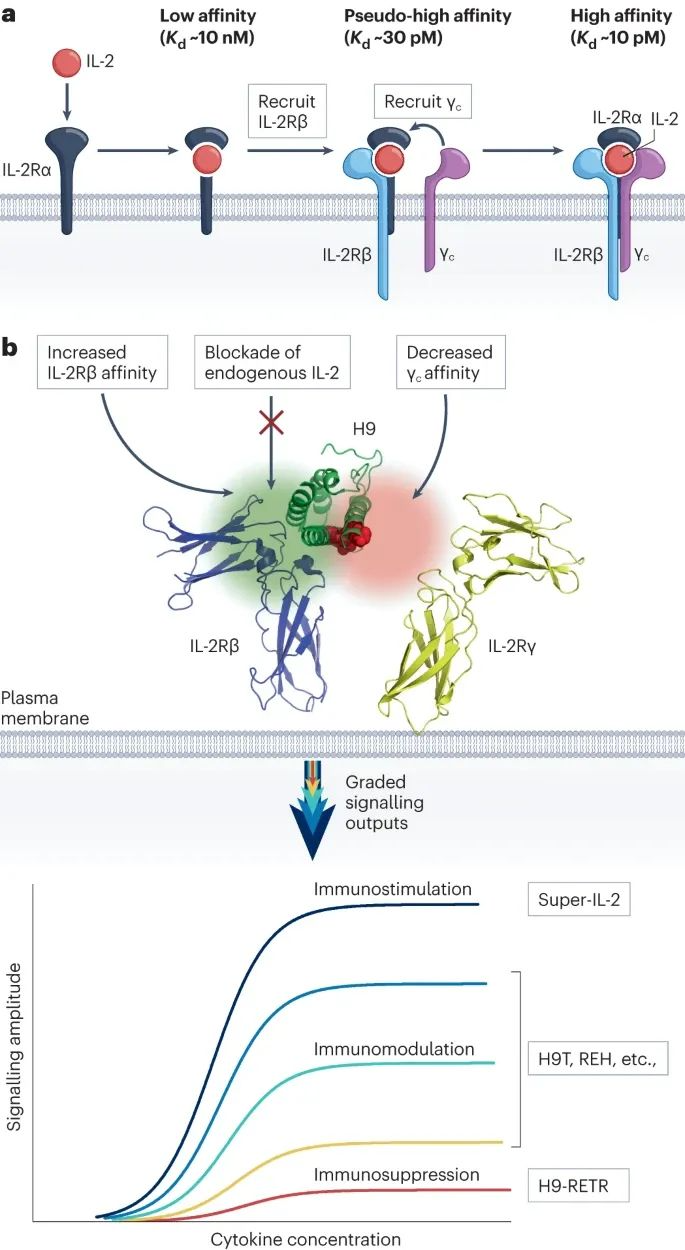
Mutations impacting the IL-2Rα or IL-2Rβ interface have diverse effects, with the specific mutation position determining the outcome. For example, in human IL-2 dimers, mutations at IL-2Rα R38, F42, Y45, and E62 stimulate normal proliferation of NK cells and CD8+ T cells but inhibit Treg cells. Conversely, mutations targeting N88 reduce IL-2Rβ interaction and selectively promote Treg cells.
IL-2 superagonists and partial agonists involve the use of IL-2 muteins with specific mutations, such as L80F, R81D, L85V, I86V, and I92F. These modified variants, known as "super IL-2s" (e.g., H9), effectively expand Teff cells without a preference for IL-2Rα. Similar effects can also be observed with IL-4.
Additionally, there are partial agonists, such as IL-10 variants, which exhibit enhanced IL-10Rβ binding. These variants have been identified through yeast surface display and demonstrate heightened potency even at lower doses in CD8+ T cells and monocytes.
Soluble cytokine receptor complexes and fusion proteins
In addition to native cytokines, soluble cytokine-receptor complexes or fusions can be utilized, leveraging the properties of specific receptors. Examples include IL-2–IL-2Rα and IL-15–IL-15Rα complexes.
Novel cytokines can be designed de novo through computational modeling of native cytokine binding sites. For instance, the newly developed protein NL-201 selectively binds to IL-2Rβ and γ, without binding IL-2Rα, thereby avoiding immunosuppression and potential side effects. Studies have demonstrated that NL-201 exhibits low toxicity and robust anti-cancer effects. However, significant deviations from native proteins in this approach may introduce antigenicity concerns.
Fusion proteins combining different cytokines enable the integration of signals from two distinct cytokines or molecules. For example, the combination of low-dose IL-2 and TNFR2 agonists can enhance Treg cells. Additionally, the immunocytokine PD1–IL-2v expands stem cell-like CD8+ T cells by binding to PD1 and IL-2Rβγ in cis, resulting in significant anti-tumor effects.
Another approach for generating cytokine-like molecules involves modular single-chain bispecific ligands, which consist of variable heavy chain antibody fragments (VHHs) and/or scFv domains. These ligands offer versatility in their design and functionality.
Strategies for Inhibiting Cytokines
Strategies for inhibiting cytokines involve various approaches aimed at modulating their activity. Antibodies against cytokines or their receptors are utilized to specifically target and inhibit their signaling and biological functions. Another key strategy is the inhibition of JAKs (Janus kinases) and STATs (Signal Transducer and Activator of Transcription). By targeting these signal molecules, the downstream effects of cytokine signaling can be effectively suppressed.
In addition to these approaches, targeted protein degraders such as PROTACs (Proteolysis-Targeting Chimeras) and KineTACs (Kinase-Targeting Chimeras) are emerging strategies for inhibiting cytokines. PROTACs are particularly suited for targeting signaling molecules like STAT3 and JAKs, leading to their degradation and subsequent inhibition of cytokine signaling.
Another innovative approach involves the use of cytokine receptor-targeting chimeras known as KineTACs. These recombinant bispecific antibodies are designed to bind both cytokines and their receptors. Upon binding, the KineTAC forms a complex that enters cells through endocytosis and undergoes degradation in lysosomes. This approach has shown success in targeting cytokines such as CXCL11, CXCL12, vMIPII, and IL-2, effectively inhibiting their activity.
By employing these strategies, researchers can modulate cytokine signaling pathways and potentially develop novel therapeutic interventions to target cytokine-related diseases and disorders.
Reference
Leonard, W.J., Lin, JX. Strategies to therapeutically modulate cytokine action. Nat Rev Drug Discov (2023).