Blue Earth Therapeutics reports encouraging findings from preclinical assessment of 225Ac-rhPSMA-10.1 of Prostate Cancer
Blue Earth Therapeutics, a subsidiary of Bracco and an emerging pioneer in the production of ground-breaking, next-generation therapeutic radiopharmaceuticals, released results from a range of preclinical studies. These were aimed at assessing the binding potency, lipophilicity, cell absorption, and therapeutic effectiveness of 225Ac-rhPSMA-10.1 in preclinical prostate cancer treatment models, with 177Lu-rhPSMA-10.1 utilized as a reference comparator. The results indicated that both 225Ac-rhPSMA-10.1 and 177Lu-rhPSMA-10.1 demonstrated excellent PSMA binding potency, superior cell absorption, and comparable lipophilicity.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
A preclinical prostate cancer model was used to assess the therapeutic efficacy and response of 225Ac-rhPSMA-10.1, which demonstrated significant tumor growth retardation compared to a control group. The findings were discussed during a verbal discourse at the Annual Congress of the European Association of Nuclear Medicine in Vienna, Austria. Blue Earth Therapeutics is leading the development of the 225Ac-rhPSMA-10.1, a prostate-specific membrane antigen targeted radiotherapeutic drug currently under investigation and acing as the primary alpha-emitting contender in the company's oncology advancement program.
David E. Gauden, CEO of the company expressed satisfaction at the premiere disclosure of preclinical results from Blue Earth Therapeutics' prostate cancer initiative using 225Ac-radiolabeled rhPSMA-10.1 at the EANM’s23 Annual Gathering. He further emphasized that the preclinical analysis showed 225Ac-rhPSMA 10.1 to have a promising treatment profile, while utilizing 1000 times less radioactive material than 177Lu-rhPSMA-10.1.
This property along with similar in-vitro attributes and therapeutic efficacy was found noteworthy. Gauden intentions are to transition this innovative alpha-targeted treatment into clinical phases. Studies aimed at enabling Investigational New Drug (IND) application have been concluded and a phase 1 clinical trial of 225Ac-rhPSMA-10.1 is scheduled to commence in the first six months of 2024.
The results were elaborated in the oral presentation titled "Preclinical Assessment of225Ac-rhPSMA-10.1, A Unique Radiohybrid PSMA Compound for Targeted Alpha Therapy for Prostate Cancer" by Caroline Foxton, Ph.D. from Blue Earth Group, Oxford, UK at the Annual Congress of the European Association of Nuclear Medicine held on September 10, 2023.
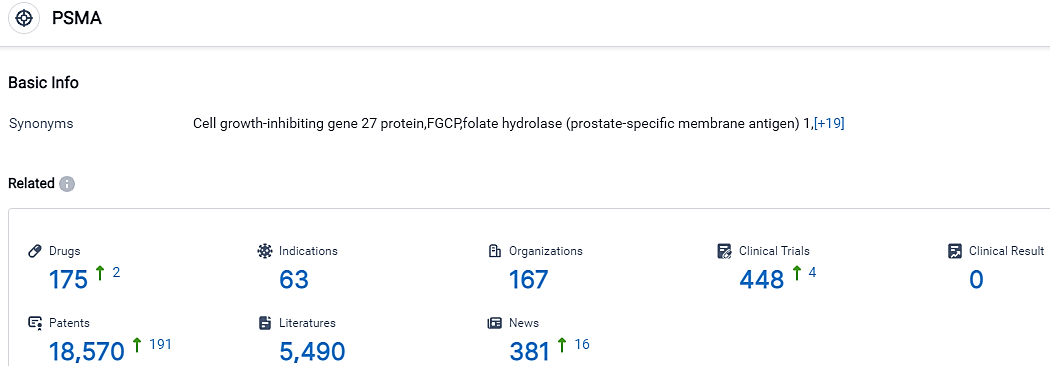
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 15, 2023, there are 175 investigational drugs for the PSMA target, including 63 applicable indications,167 R&D institutions involved, with related clinical trials reaching 448,and as many as 18570 patents.
225Ac-rhPSMA-10.1 is a therapeutic radiopharmaceutical being developed by Blue Earth Therapeutics Ltd. It targets PSMA and is intended for the treatment of prostatic cancer. While it is currently in the preclinical phase, its potential to deliver targeted radiation to cancer cells makes it a promising candidate for further research and development.