Decoding Vilobelimab: A Comprehensive Study of its R&D Trends
Vilobelimab's R&D Progress
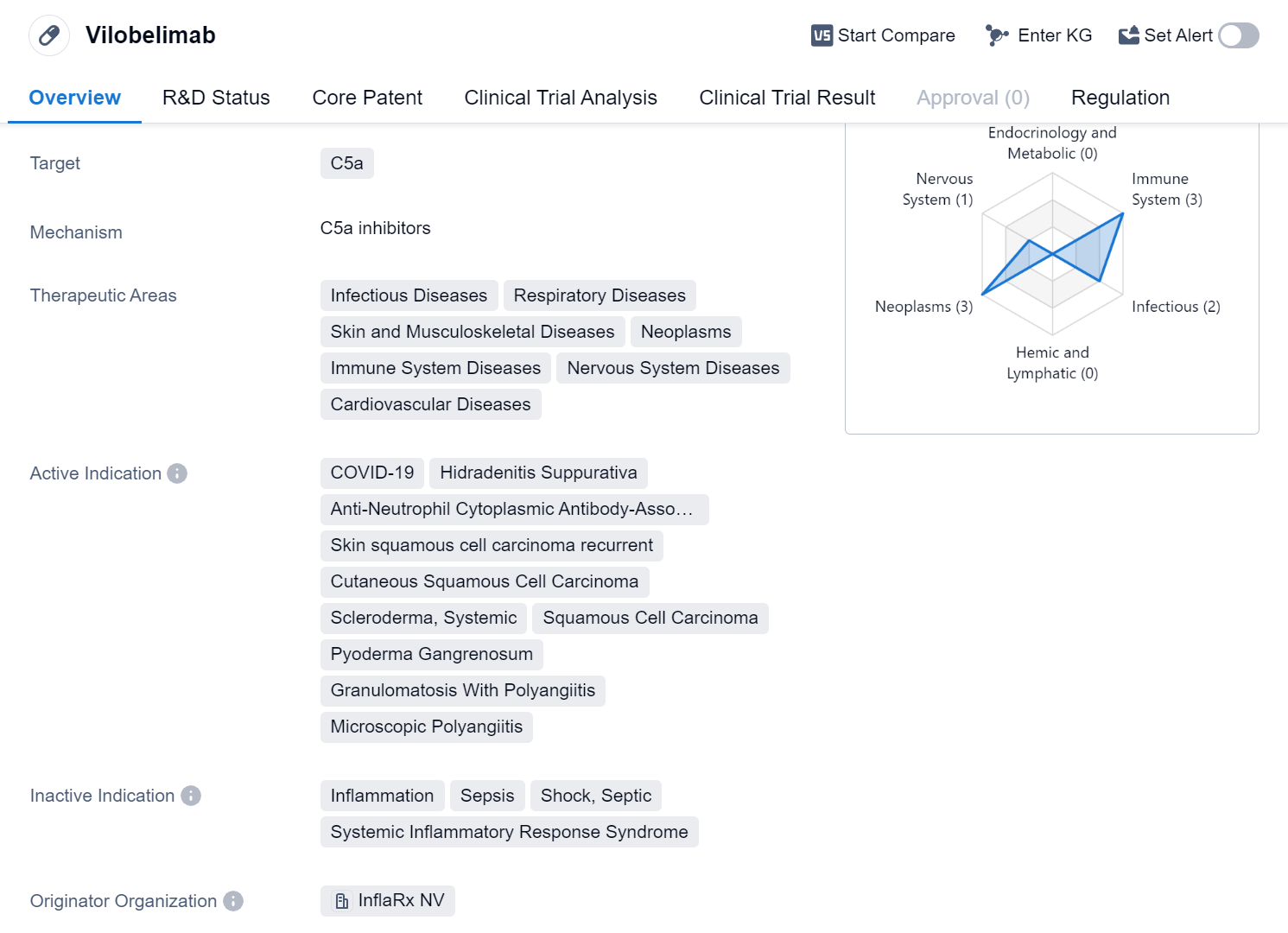
Vilobelimab is a monoclonal antibody drug developed by InflaRx NV. It falls under the category of biomedicine and specifically targets C5a, a protein involved in the immune response. The drug has shown potential in treating various therapeutic areas, including infectious diseases, respiratory diseases, skin and musculoskeletal diseases, neoplasms, immune system diseases, nervous system diseases, and cardiovascular diseases.
Vilobelimab has been indicated for several active conditions, including COVID-19, Hidradenitis Suppurativa, Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis, recurrent skin squamous cell carcinoma, cutaneous squamous cell carcinoma, systemic scleroderma, squamous cell carcinoma, pyoderma gangrenosum, granulomatosis with polyangiitis, and microscopic polyangiitis.
The drug has reached the highest phase of development, which is approval. It is expected to receive its first approval globally in April 2023, with the United States being the first country/location to grant approval. Vilobelimab has undergone regulatory processes such as Fast Track designation, Emergency Use Authorization, and Orphan Drug designation.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Vilobelimab: C5a inhibitors
C5a inhibitors are a type of medication that target and inhibit the activity of the C5a protein. C5a is a component of the complement system, which is a part of the immune system responsible for defending the body against infections. When the complement system is activated, C5a is released and plays a role in inflammation and recruitment of immune cells to the site of infection.
C5a inhibitors work by blocking the activity of C5a, thereby reducing inflammation and preventing excessive immune cell activation. These inhibitors can be used in the treatment of various inflammatory conditions, such as rheumatoid arthritis, lupus, and certain kidney diseases.
From a biomedical perspective, C5a inhibitors are a promising therapeutic approach for modulating the immune response and controlling inflammation. By specifically targeting C5a, these inhibitors can help regulate the immune system without completely suppressing it, which is beneficial for patients with chronic inflammatory diseases.
Drug Target R&D Trends for Vilobelimab
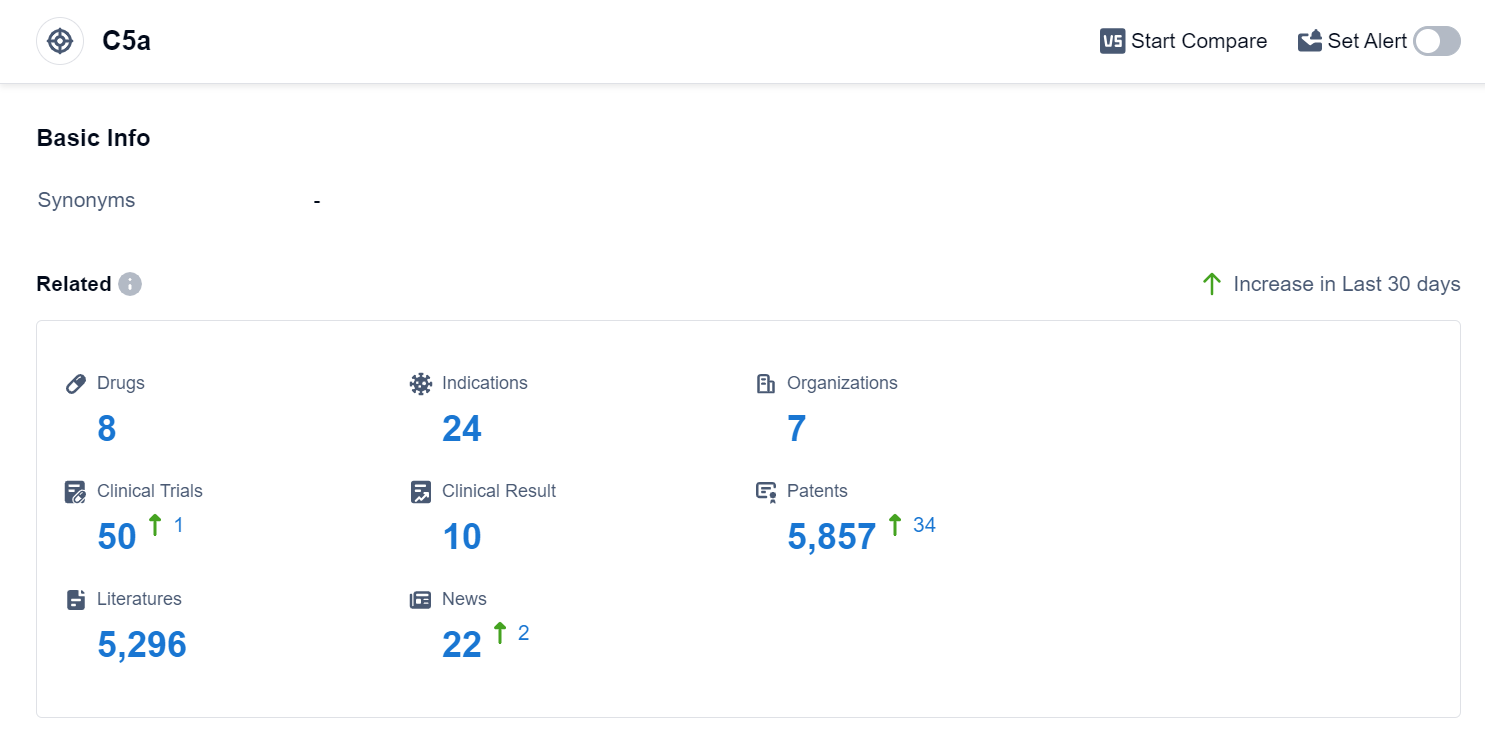
According to Patsnap Synapse, as of 5 Sep 2023, there are a total of 8 C5a drugs worldwide, from 7 organizations, covering 24 indications, and conducting 50 clinical trials.
The analysis of target C5a reveals that InflaRx NV is the company with the highest number of drugs in various stages of development. They are actively involved in R&D for drugs targeting C5a. Several drugs have been approved for indications such as COVID-19, Hidradenitis Suppurativa, and various autoimmune diseases. Monoclonal antibodies are the most rapidly progressing drug type, with the presence of biosimilars indicating intense competition. The countries/locations developing fastest under the target C5a include the United States, European Union, Germany, and China. The competitive landscape for target C5a is dynamic, with multiple companies and countries actively involved in R&D for drugs targeting this pathway. Further research and development are needed to fully understand the future development and potential of target C5a in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Vilobelimab is a monoclonal antibody drug developed by InflaRx NV that targets C5a. It has shown potential in treating various therapeutic areas, including infectious diseases, respiratory diseases, and neoplasms. The drug has received approval and is expected to be first approved in the United States in April 2023. It has undergone regulatory processes such as Fast Track designation, Emergency Use Authorization, and Orphan Drug designation.