Migraine Treatment Medication——CGRP antagonists
CGRP, or calcitonin gene-related peptide, is a neuropeptide that plays a crucial role in the human body. It is primarily found in the nervous system and acts as a potent vasodilator, meaning it widens blood vessels. CGRP is involved in various physiological processes, including regulating blood flow, modulating pain transmission, and promoting neurogenic inflammation. Additionally, it has been implicated in migraine headaches, as elevated levels of CGRP have been observed during migraine attacks. Understanding the role of CGRP has led to the development of targeted therapies that aim to block its activity, providing potential relief for individuals suffering from migraines and other related conditions.
CGRP Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 10 Sep 2023, there are a total of 19 CGRP drugs worldwide, from 36 organizations, covering 20 indications, and conducting 194 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.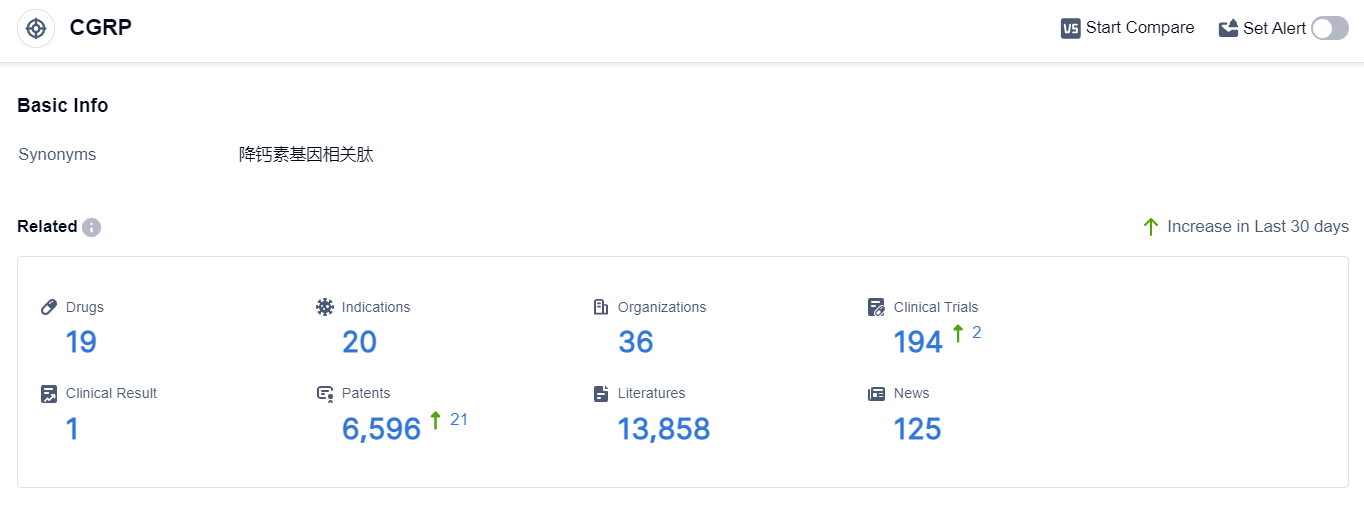
The current competitive landscape for the target CGRP is characterized by the presence of multiple companies with drugs in various stages of development. Eli Lilly & Co., Lundbeck Foundation, Pfizer Inc., Teva Pharmaceutical Industries Ltd., and AbbVie, Inc. are the companies growing fastest under this target.
The primary indication for drugs targeting CGRP is Migraine Disorders, with several drugs approved for this indication. Monoclonal antibodies and Small molecule drugs are the drug types progressing most rapidly, indicating intense competition.
The United States, European Union, and Canada are leading in terms of drug development, with China showing some progress. Overall, the target CGRP has a promising future in the pharmaceutical industry, with potential advancements in the treatment of migraine and other related disorders.
Key Drug: Vazegepant
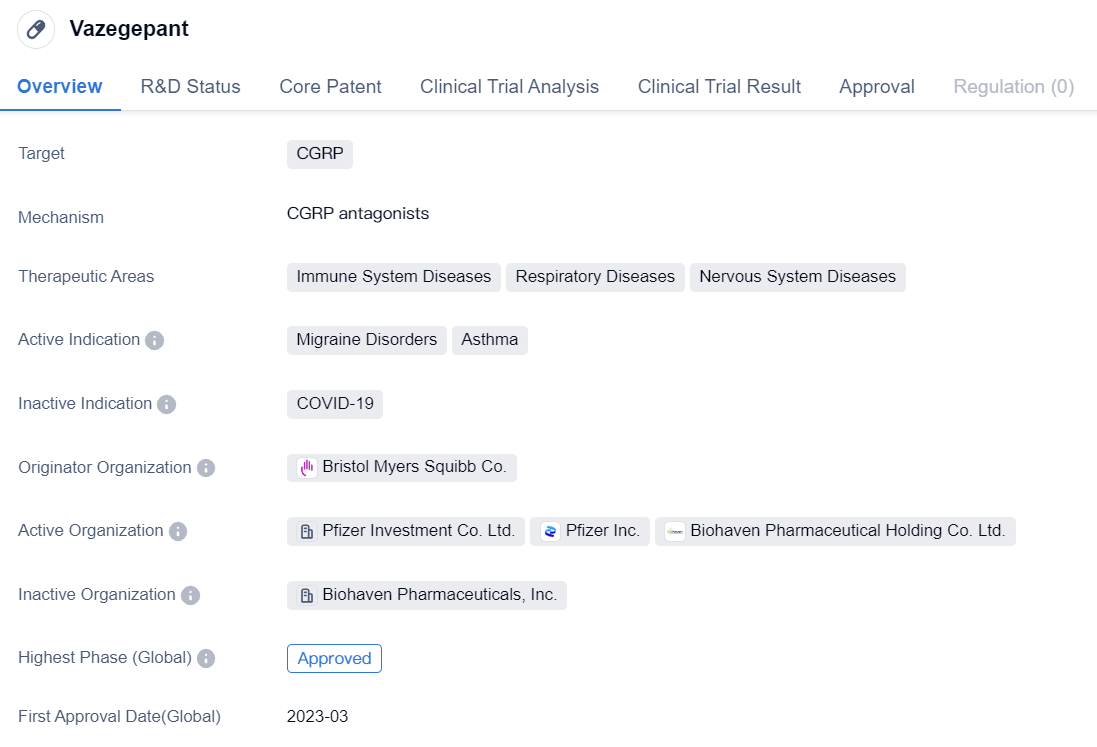
Vazegepantis a small molecule drug that targets CGRP. It is primarily used for the treatment of immune system diseases, respiratory diseases, and nervous system diseases. The drug has been approved for the treatment of migraine disorders and asthma.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Vazegepant is developed by Bristol Myers Squibb Co., a renowned pharmaceutical company. It has reached the highest phase of development globally, which is the approved stage. This indicates that the drug has successfully completed all necessary clinical trials and has been granted regulatory approval for use in patients. The first approval of Vazegepant was granted in the United States in March 2023.
In addition to its global approval, Vazegepant is also undergoing phase 3 clinical trials in China. Phase 3 trials are conducted to further evaluate the safety and efficacy of a drug in a larger population before seeking regulatory approval. This suggests that the drug is being tested in Chinese patients and may potentially be approved for use in China in the future.
The therapeutic areas of Vazegepant encompass a wide range of diseases. Immune system diseases, such as autoimmune disorders, are characterized by an overactive immune response that can lead to chronic inflammation and tissue damage. Respiratory diseases, including asthma, affect the airways and can cause symptoms like wheezing, coughing, and shortness of breath. Nervous system diseases involve the brain, spinal cord, and nerves, and can manifest as conditions like migraines.
The approval of Vazegepant for the treatment of migraine disorders and asthma highlights its potential efficacy in managing these conditions. Migraine disorders are characterized by recurrent headaches, often accompanied by other symptoms like nausea and sensitivity to light and sound. Asthma is a chronic respiratory condition characterized by inflammation and narrowing of the airways, leading to breathing difficulties.
Overall, Vazegepant is a small molecule drug developed by Bristol Myers Squibb Co. that targets CGRP. It has been approved for the treatment of migraine disorders and asthma, with its first approval granted in the United States. The drug is also undergoing phase 3 trials in China, indicating its potential for future approval in the Chinese market. With its therapeutic areas covering immune system diseases, respiratory diseases, and nervous system diseases, Vazegepant holds promise in addressing a range of medical conditions.
Atogepant
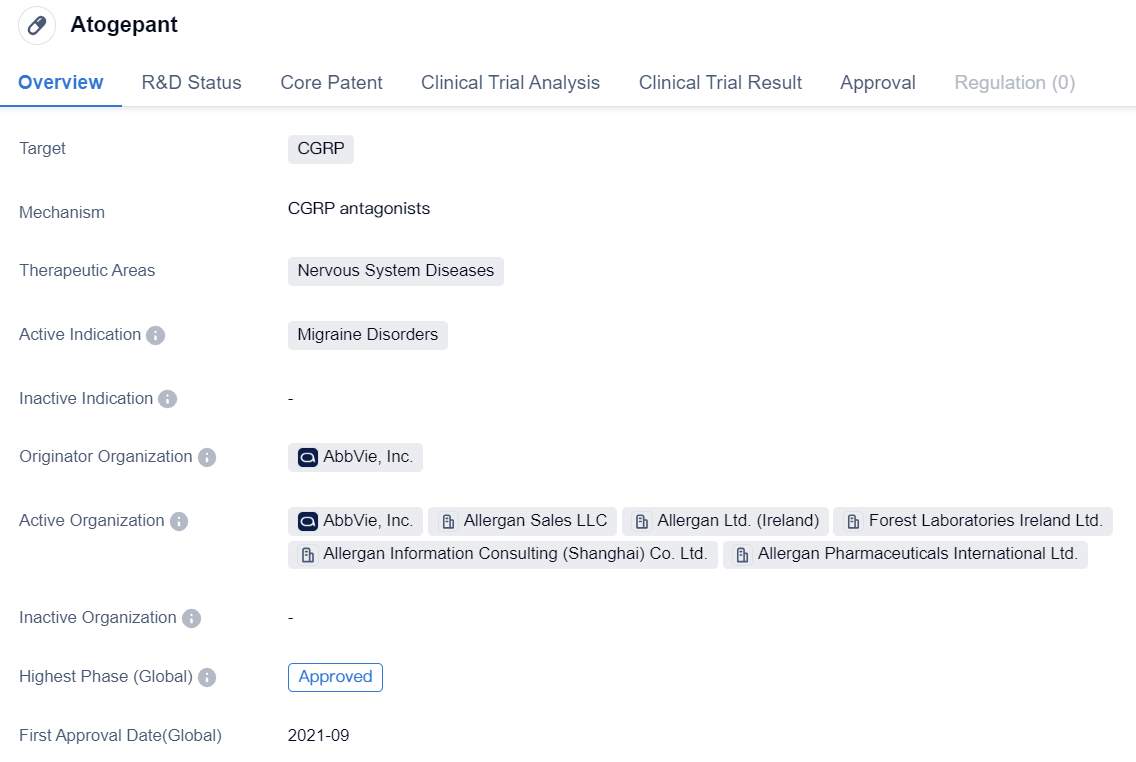
Atogepant is a small molecule drug that falls under the therapeutic area of Nervous System Diseases. It specifically targets CGRP, making it a potential treatment for Migraine Disorders. The drug has been developed by AbbVie, Inc., a renowned pharmaceutical organization.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As of the latest available information, Atogepant has reached the highest phase of development, which is the approval stage, on a global scale. This indicates that the drug has successfully completed all necessary clinical trials and has been granted approval for use. The first approval for Atogepant was granted in the United States in September 2021, marking a significant milestone for the drug.
In China, Atogepant has reached the Phase 3 stage, which suggests that it is currently undergoing advanced clinical trials in the country. Phase 3 trials are typically conducted to evaluate the drug's safety and efficacy in a larger patient population before seeking regulatory approval.
The specific indications for Atogepant are focused on Migraine Disorders. Migraine is a neurological condition characterized by severe headaches, often accompanied by other symptoms such as nausea, sensitivity to light, and sound. By targeting CGRP, Atogepant aims to alleviate the symptoms associated with migraines and potentially provide relief to patients suffering from this condition.