Boston Pharma unveils positive Phase 2a data for monthly NASH drug BOS-580, highlighting effects on diabetics, at 2023 AASLD Liver Meeting
Boston Pharmaceuticals, a biopharma organization in the clinical testing phase, specializing in the creation of distinct molecules targeted at major liver complications, has revealed fresh insights on BOS-580. It is an experimental, enduring analog of fibroblast growth factor 21 (FGF21), designed for managing non-alcoholic steatohepatitis.
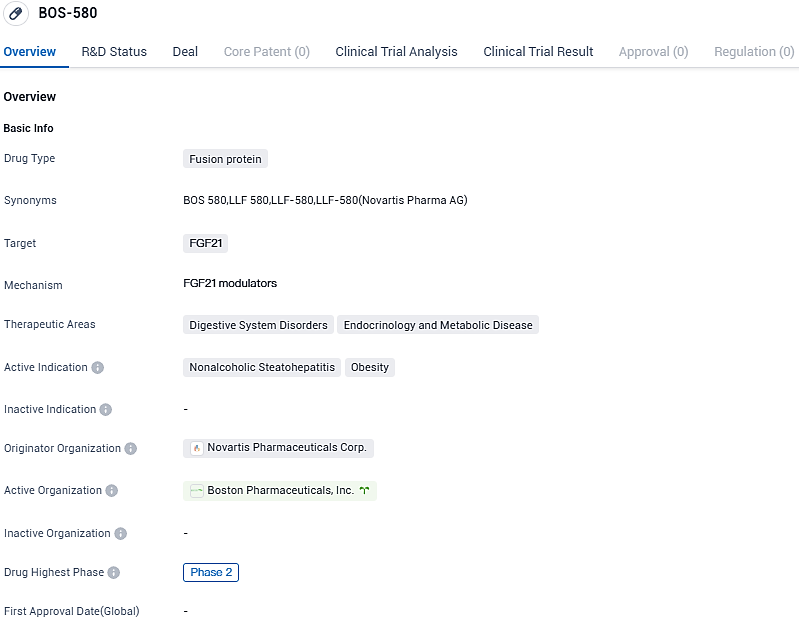
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
BOS-580, a refined FGF21 analog with prolonged action, is delivered via a single subcutaneous injection every month. It is especially designed to diminish liver fat, liver inflammation shown by liver damage and fibrosis biomarkers, and boost metabolism, as demonstrated by the effect on metabolic biomarkers, in NASH patients.
Presenting these findings at the AASLD, Boston Pharmaceuticals CEO Sophie Kornowski, PharmD, expressed her belief that BOS-580's potential for monthly administration could offer comprehensive disease control with a good safety record for NASH patients.
"We're currently assessing a monthly administration schedule for BOS-580 and its impact on individuals living with NASH and liver fibrosis. We plan to promptly forge ahead with our development efforts and commence significant clinical studies," Kornowski continued to state.
Study results suggest that individuals with non-alcoholic fatty liver disease, a potential precursor to NASH, have a greater likelihood of developing type 2 diabetes, and that two out of every three Individuals with type 2 diabetes also suffer from NAFLD. Recognized by the AASLD as a standout poster, this study probed into the effects of BOS-580 treatment on glycemic control markers and liver steatosis reduction in diabetic sub-groups participating in a randomized, double-blind, placebo-controlled Phase 2a study that involved phenotypic NASH patients.
Professor Rohit Loomba, M.D., MHSc, Division Chief of Gastroenterology and Hepatology, and MASLD Research Center Director at the University of California, San Diego revealed that: “BOS-580 treatment proved promising in managing healthy HbA1c levels in phenotypic NASH patients, regardless of their concurrent type 2 diabetes condition. Our findings indicated that BOS-580 consistently performed well throughout all diabetic sub-groups involved in the Phase 2a clinical study."
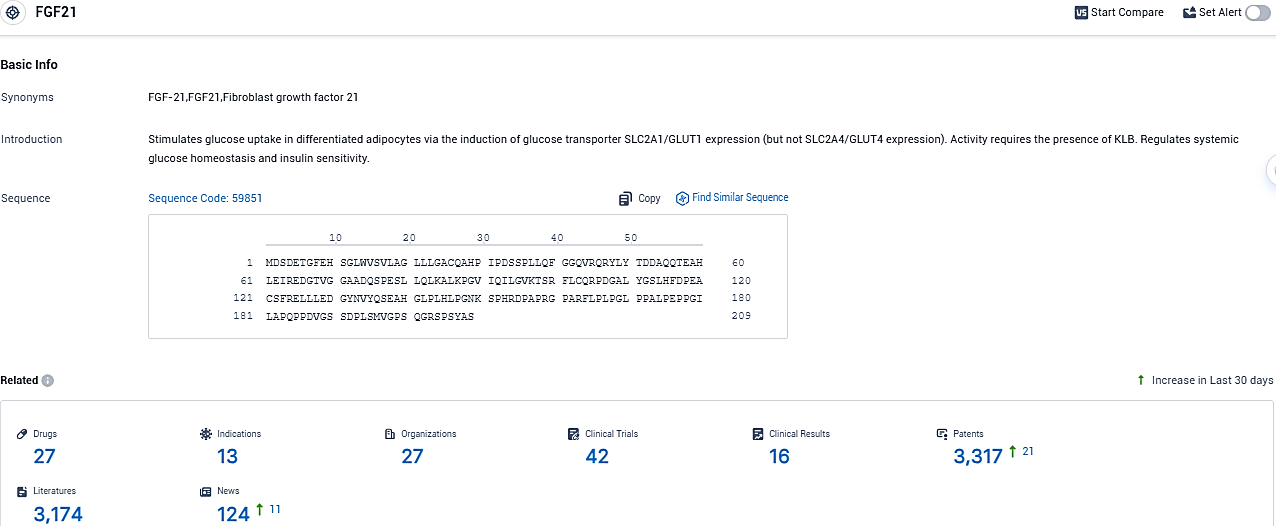
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 18, 2023, there are 27 investigational drugs for the FGF21 target, including 13 indications, 27 R&D institutions involved, with related clinical trials reaching 42, and as many as 3317 patents.
BOS-580 shows promise as a potential treatment for NASH and obesity. Its fusion protein nature and targeting of FGF21 highlight its innovative approach to addressing these conditions. However, further clinical trials and regulatory approvals will be necessary to determine its ultimate efficacy and safety profile. The development of BOS-580 represents an important advancement in the field of biomedicine, offering hope for patients suffering from digestive system disorders and endocrinology and metabolic diseases.