Merck Enhances Cancer Drug Offering By Securing Deal with Abbisko for Market Launch of Advanced Stage Drug, Pimicotinib
The prominent science and technology corporation, Merck, has declared a formal licensing contract with the Shanghai-based firm Abbisko Therapeutics Co. Ltd. The agreement focuses on the molecule pimicotinib (ABSK021), which is under investigation in a critical Phase III clinical trial, aiming at addressing the medical condition referred to as tenosynovial giant cell tumor (TGCT).
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
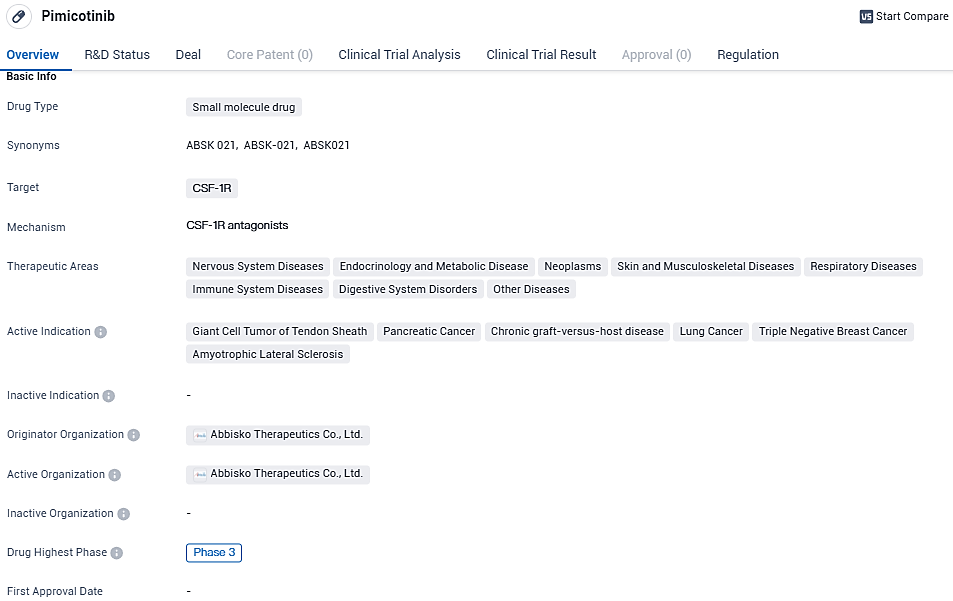
TGCT presents as a non-malignant growth within joint spaces, leading to symptoms such as localized enlargement, discomfort, joint rigidity, and a decrease in joint movement. The therapeutic strategies to manage this condition, which can impinge significantly on the well-being of sufferers, are scant. In a move to extend patient care, Merck has been afforded rights to market pimicotinib across several regions including mainland China, Hong Kong, Macau, and Taiwan, with an additional provision for global distribution rights.
"This collaboration with Abbisko catalyzes our potential to bring breakthrough therapeutic options to a demographic in dire need within China, and perhaps internationally," mentioned Andrew Paterson, the Senior Marketing Executive of Merck's Healthcare division. "By introducing pimicotinib, we confront a critical gap in healthcare provisioning, while growing our presence in the oncological sector of China, recognized as the world's second most substantial pharma market."
The experimental drug pimicotinib, taken orally, exhibits strong selectivity and potency as a CSF-1R inhibitor and is undergoing testing in a worldwide Phase III clinical study aimed at determining its efficacy for TGCT. To date, China has not sanctioned any medicinal solutions for TGCT, with only a single therapeutic approved for this indication in the U.S.
Dr. Xu Yao-chang, Abbisko Therapeutics’ Chairman, stated, "Our alliance with Merck represents a key progress point for propelling pimicotinib towards global market access and reflects a fresh paradigm for commercial strategies for Abbisko’s product pipeline moving forward. We're excited to partner with such a prominent global pharma entity, working side by side to hasten the international regulatory approval and market introduction of pimicotinib."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
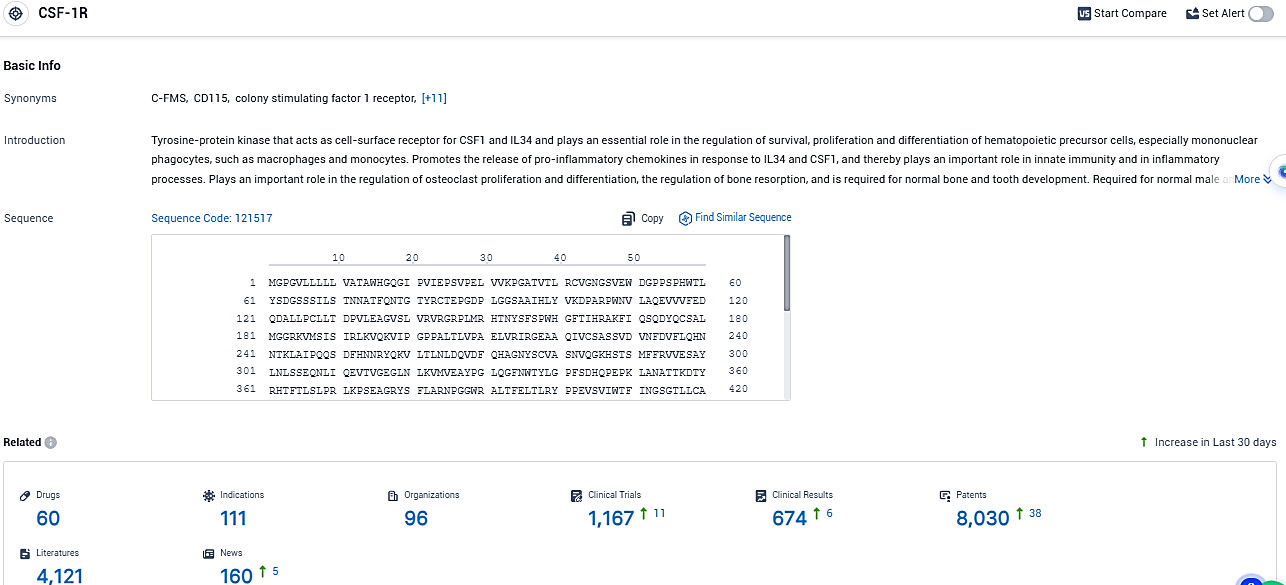
According to the data provided by the Synapse Database, As of December 12, 2023, there are 60 investigational drugs for the CSF-1R target, including 111 indications, 96 R&D institutions involved, with related clinical trials reaching 1167, and as many as 8030 patents.
Pimicotinib (ABSK021), which was independently developed by Abbisko Therapeutics, is a novel, orally administered, highly selective and potent small-molecule inhibitor of CSF-1R. Pimicotinib has been granted breakthrough therapy designations by China National Medical Products Administration and the U.S. Food and Drug Administration and priority medicine designation from the European Medicines Agency for the treatment of patients with TGCT that are not amenable to surgery.