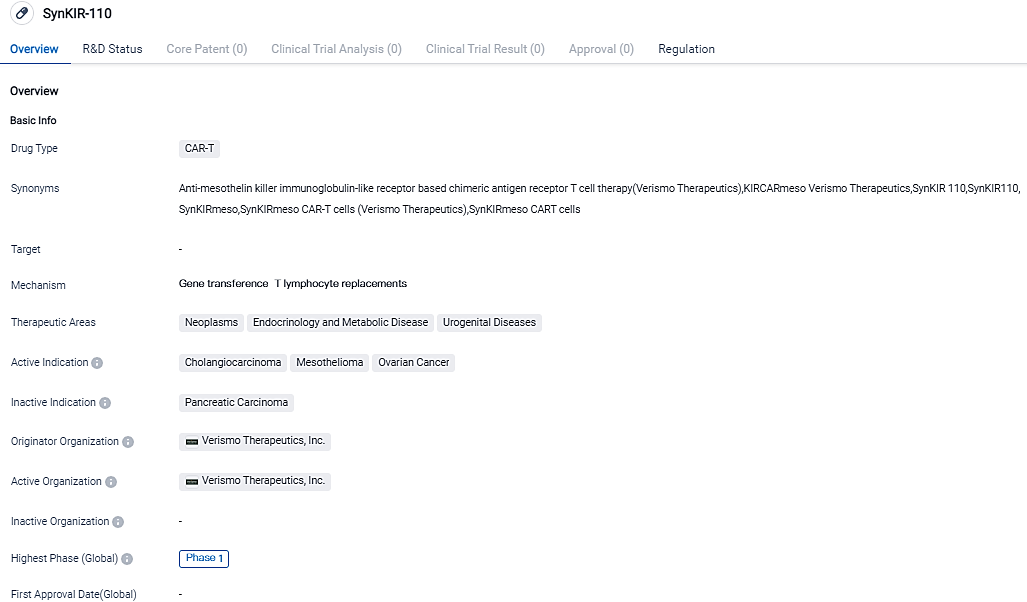
Verismo Therapeutics provides SynKIR-110 treatment for the first advanced cancer patient in clinical trials
Verismo Therapeutics, a clinical-stage company specializing in CAR-T therapies, has initiated the dosing of the first patient in their STAR-101 clinical trial. The trial aims to evaluate the efficacy of the SynKIR-110 product in individuals diagnosed with advanced ovarian cancer, mesothelioma, and cholangiocarcinoma.
Verismo Therapeutics has developed this innovative therapy based on the KIR-CAR platform technology, which was invented at and licensed from the University of Pennsylvania.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The Phase 1 clinical trial is an important step in developing better treatment options for patients with solid tumors that express mesothelin. The trial, called STAR-101, will assess the safety, feasibility, and potential activity of SynKIR-110, a product made up of autologous T cells that have been modified to target mesothelin. SynKIR-110 has the potential to be a new treatment option for ovarian cancer, mesothelioma, and cholangiocarcinoma, all of which express mesothelin.
Dr. Bryan Kim, the Co-Founder and CEO of Verismo Therapeutics, is excited about this milestone and believes it will provide much-needed treatment options for patients with solid tumors that express mesothelin.
The trial will consist of an intervention period where participants will receive chemotherapy followed by a single infusion of SynKIR-110, as well as a 12-month follow-up period.
The KIR-CAR platform is a dual-chain CAR T cell therapy that has shown promise in animal models, even in challenging solid tumor environments. The addition of DAP12 as a costimulatory molecule enhances T cell activity and improves the persistence of the KIR-CAR therapy.
The KIR-CAR platform is being studied in combination with other emerging technologies like gene engineering, advanced cell manufacturing, combinational therapies, and allogeneic cellular therapies to potentially provide a next-generation targeted immunotherapy for patients in need.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
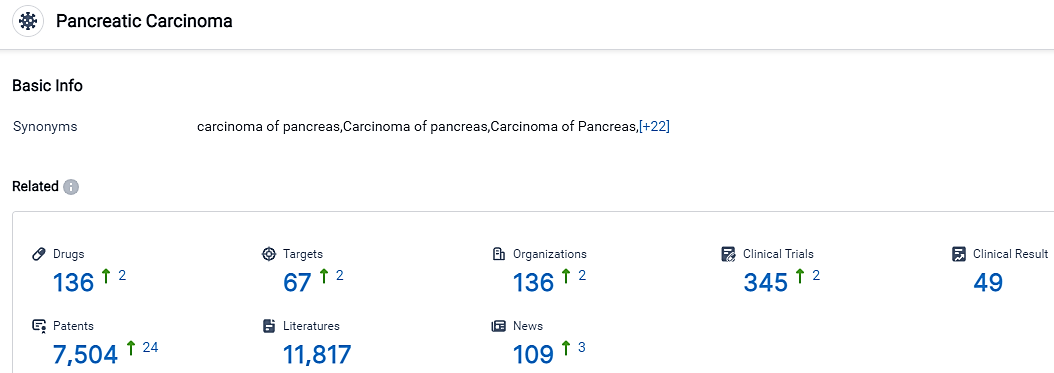
According to the data provided by the Synapse Database, As of September 11, 2023, there are 136 investigational drugs for Pancreatic Carcinoma, including 67 targets, 136 R&D institutions involved, with related clinical trials reaching 345, and as many as 7504 patents.
The increasing interest in the treatment options for Pancreatic Carcinoma indicates a rising enthusiasm in advancing research and development for this disease. Companies like Roche Holding AG, Merck & Co., Inc., and Pfizer Inc. have made notable advancements in their efforts to develop new treatments. The identification of targets such as TOP1 and TYMS offers potential areas of focus for future research and development. The development of new treatments for Pancreatic Carcinoma holds great promise for improving patient outcomes.