Can JAK3 Replicate TYK2's Success?
Janus Kinase Plays a Critical Role in Immune Responses by Transmitting Signals from Over 50 Cell Factors, Making It an Attractive Therapeutic Target for Autoimmune Diseases. However, Currently Available JAK Inhibitors are Accompanied by Some Adverse Reactions Due to Their Lack of Selectivity in Inhibiting Cytokine Signals. Therefore, The Next Generation of Inhibitors Need to Avoid Adverse Events While Maintaining Therapeutic Effectiveness. Among the JAK Family Members, JAK3, which Only Regulates a Narrow Spectrum of γc Cytokines, Emerges as a Potential Ideal Target.
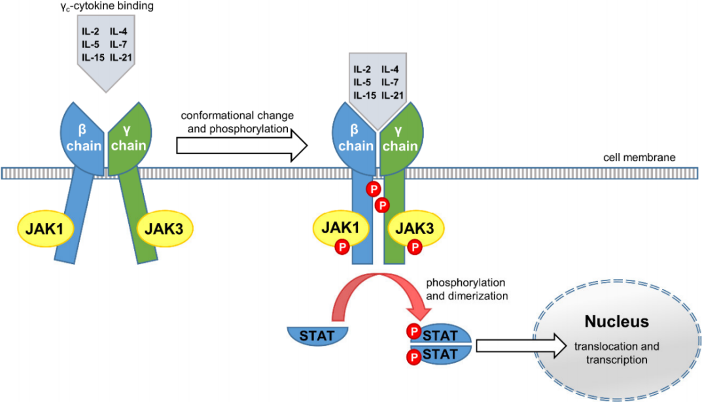
In the Janus kinase family of non-receptor tyrosine kinases, there are four subtypes, JAK1, JAK2, JAK3, and TYK2 (Tyrosine Kinase 2), which send out signals through the JAK/STAT pathway. JAK1, JAK2, and TYK2 are ubiquitously expressed, while JAK3 is mainly expressed in lymphoid tissues and is a selective regulator in lymphocyte development, playing roles in the immune system that make JAK3 an attractive target for treating autoimmune diseases. JAK3 selectively binds to a single subunit of cytokine receptors, called common γ-subunit or γc chain. JAK3, in conjunction with JAK1, is involved in signal transmission initiated by 6 cytokines (interleukin-2, -4, -5, -7, -15, and -21), which are essential in immune homeostasis. An example of the key role of JAK3 in autoimmune diseases is X-linked severe combined immunodeficiency (X-SCID). Mutations in JAK3 or γc cytokines can result in X-SCID, a pediatric emergency condition that leads to severe infections and diarrhea and requires immediate hematopoietic stem cell transplantation (HSCT) or gene therapy.
The Discovery Process and Structural Characteristics of JAK3 Inhibitors
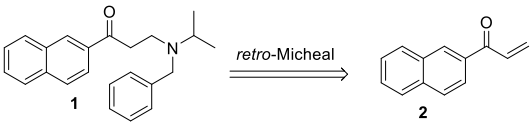
A difference in the amino acid sequence between JAK3 and other JAK members is the presence of a cysteine residue in the ATP binding pocket, which is replaced by a serine residue in the other three subtypes, providing a structural biology basis for designing highly selective JAK3 inhibitors. In 2000, Brown and others at AstraZeneca first reported the discovery process of JAK3 selective inhibitors, initially using enzyme-linked immunosorbent assay (ELISA) to conduct high-throughput screening of >200,000 compounds at a test concentration of 10µM. A series of 2-aminoethyl ketone (AEKs) compounds were synthesized, including the most effective compound 1 (JAK3 IC50 80nM, [ATP] = 2µM). It was found however, under experimental conditions, compound 1 could decompose, and it had previously been reported that AEKs could undergo reverse Michael addition reaction. After preparing and evaluating more stable AEKs, their JAK3 activity was found to be lower, possibly due to the AEKs decomposing under ELISA experimental conditions, generating highly active naphthyl ethylene ketone, which might interact covalently with JAK3 protein. When the most effective compound 1 was found to have a half-life of 36 minutes under experimental conditions and the possible decomposition product was 2, this was confirmed. Compound 2 was subsequently tested, revealing its JAK3 IC50 also as 80nM ([ATP] = 2µM), demonstrating that the decomposition product was actually the active inhibitor.
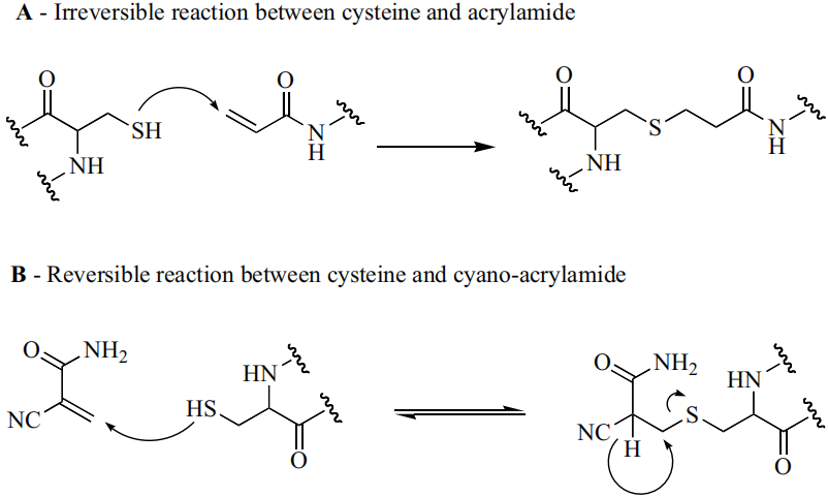
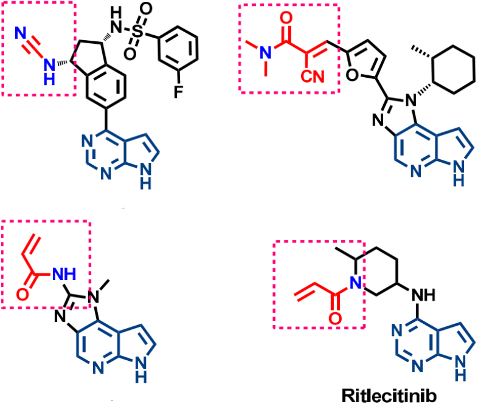
Subsequent JAK3 Selective Inhibitors Retain the Enone Fragment: Differential Effects of Cyanide Introduction on Reversibility, Increased Acidity of Cα Hydrogens, Product Instability, and Conversion of Irreversible Cysteine Addition to Reversible Addition. The Advantages of Reversible Covalent Inhibitors include Prolonged Inhibition Duration, Lower Effective Concentration, and Reduced Off-Target Toxicity.
Ritlecitinib is an irreversible inhibitor of JAK3. As of March 2023, according to the information obtained from Synapse using the keyword 'Ritlecitinib', the phase II clinical trials for both monotherapy and combined therapy for rheumatoid arthritis have failed. The ulcerative colitis indication is in phase III clinical trials, Crohn's disease is in phase II clinical trials, and Pfizer plans to initiate a phase II clinical trial (NCT05743244) for type I diabetes in 2023. This is currently the fastest progressing JAK3 selective inhibitor.
The inhibitory activity of Ritlecitinib is mediated by its interaction with the Cys909 residue in JAK3. Covalent binding enhances the compound's selectivity for JAK3. Ritlecitinib inhibits JAK3 with an IC50 of 33.1 nM, and exhibits no activity against JAK1, JAK2, and TYK2 (IC50>10,000 nM). It also suppresses Th1 and Th17, achieving this by inhibiting the production of IFNγ (IC50=48 nM) and IL-17 (IC50=269 nM).
JAK inhibitors treat IBD by blocking communication between immune cells. In May 2018, the FDA approved tofacitinib for the treatment of moderate to severe UC. Tofacitinib is an oral small molecule inhibitor, which inhibits JAK1 and JAK3, disrupting the downstream JAK/STAT signaling pathway, thereby regulating DNA transcription. The selective JAK1 inhibitor itacitinib has shown good therapeutic effects in patients with aGVHD, but due to side effects, several indications such as pancreatic cancer, plaque psoriasis, and rheumatoid arthritis have been discontinued. Whether selective JAK3 inhibitors can reduce the side effects caused by broad inhibition of cytokines has been validated by at least the phase I clinical data from ritlecitinib. No major adverse cardiovascular events (MACE), deaths, or opportunistic infections were observed in the study.
Current Status of Target JAK3
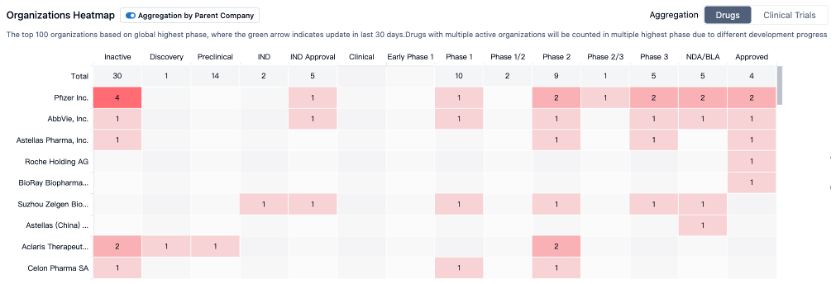
According to Synapse, at this stage, nearly 50 varieties of this target (including multiple targets) are distributed in "pre-clinical to various stages of clinical trials", developing almost 74 types of indications and the focus of clinical trials points towards autoimmune diseases (particularly rheumatoid arthritis and enteritis). The focus of clinical trials is primarily on Phase I (single target), and the number of related patents exceeds 5300 (comprehensive target).
Summary
Results from cellular experiments have repeatedly proven that, beyond the JAK1/JAK3 heterodimer pairs, the solitary inhibition of JAK3 is necessary for cancelling the downstream signal of γc chain cytokines. Covalent inhibitors of JAK3 also demonstrate stability in human whole blood and mouse liver microsomes. This stability allows certain compounds to show treatment effects in in vivo inflammation models after oral administration.
Pfizer's Ritlecitinib is presently the most advanced. Though the clinical trials for Rheumatoid Arthritis have failed, clinical trials for Ulcerative Colitis are still in progress, and new explorations in stage II clinical trials for Type I Diabetes have commenced.
However, difficulties persist. Rigel's R348, targeting ophthalmic diseases, has been declared a failure. Creabilis SA's CT 340 has made no progress in phase II clinical trials. Theravance's compound, TD-5202, entered clinical trials as early as 2020, but reports of adverse reactions during phase I trials were revealed at the 28th United European Gastroenterology Conference. Currently, it remains in Phase I. In February of this year, Theravance announced a temporary halt in the development of inhaled JAK inhibitors and layoffs of 17%, casting a shadow over TD-5202's future. OST-122, capable of inhibiting both JAK3 and TYK2, is presently progressing normally in Phase II.
Last September, the US and Japanese regulatory authorities approved the TYK2 inhibitor Deucravacitinib for the treatment of moderate to severe plaque psoriasis. This oral medication from Bristol Myers Squibb is also the first selective inhibitor of the TYK2 signaling pathway to be approved. The successful launch of Deucravacitinib once again validates the druggability of the JAK/STAT pathway. The functions of the JAK kinase in this pathway, which has been studied for 30 years, are becoming increasingly clear. Drugs have advanced from early pan inhibitors to highly selective inhibitors that target two subtypes or a single subtype. The success of JAK3 selective inhibitors in obtaining market approval, like TYK2 inhibitors, remains to be verified with time.

Reference
1.Stefan A. Laufer,et al, Recent Developments in JAK3 Inhibition: Isoform Selectivity by Covalent Targeting of Cys909.http://dx.doi.org/10.1016/j.bmcl.2017.07.079.
2.Glynn Addison,et al, Current Status in the Discovery of Covalent Janus Kinase 3 (JAK3) Inhibitors.Mini-Reviews in Medicinal Chemistry, 2019, 19, 1531-1543.
3.Hans-Günter Zerwes,et al, Selective inhibitors of the Janus Kinase Jak3 – are they effective?http://dx.doi.org/10.1016/j.bmcl.2014.08.046.