Capstan Therapeutics to Present CPTX2309 Preclinical Results at 2024 ACR Convergence
Capstan Therapeutics, Inc. ("Capstan"), a biotech firm focused on in vivo cell reprogramming via RNA delivery with their CellSeeker™ targeted lipid nanoparticle (tLNP) technology platform, has revealed plans to showcase preclinical data for CPTX2309, their premier in vivo CAR-T candidate, at the American College of Rheumatology (ACR) Convergence 2024. This event is scheduled to occur from November 14-19, 2024 in Washington, D.C.
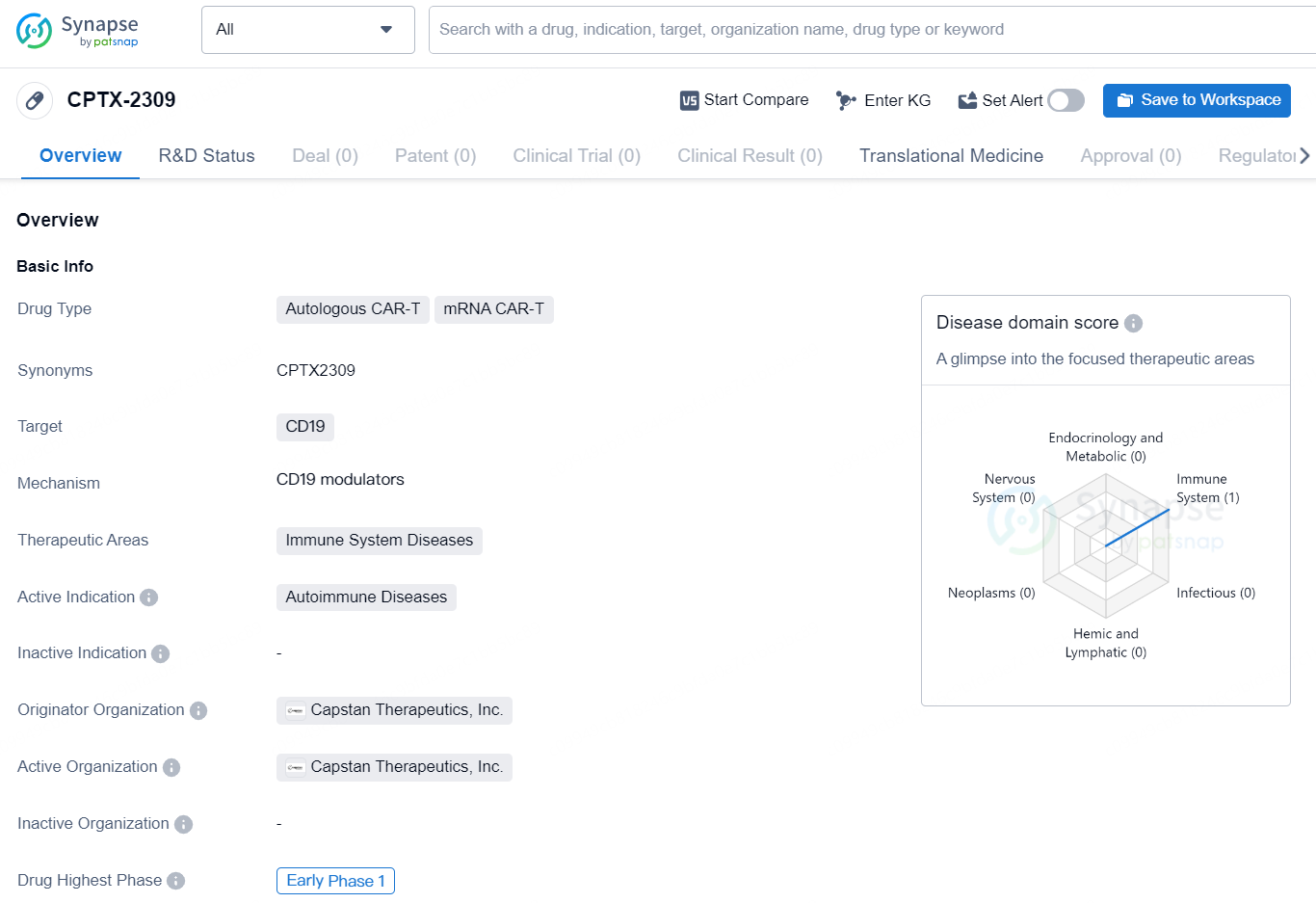
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
CPTX2309, developed from Capstan’s CellSeeker™ platform, introduces an mRNA payload encoding an anti-CD19 CAR to selectively reprogram CD8-positive T cells. This therapeutic strategy aims to reset the immune system by achieving rapid and profound depletion of B cells in both blood and lymphoid tissues, while circumventing the limitations associated with traditional ex vivo CAR-T therapies.
“Capstan’s innovative non-viral in vivo CAR-T methodology aims to combine the exceptional potency of CAR-T therapy with the convenience, pharmacologic adjustability, and scalability typical of conventional biologics,” remarked Adrian Bot, M.D., Ph.D., Chief Scientific Officer and Executive Vice President of R&D at Capstan. “The data unveiled at the conference will support the advancement of CPTX2309 for a potentially extensive array of B cell-mediated autoimmune disorders, with the goal of resetting the patient’s immune system to achieve a lasting, drug-free clinical response.”
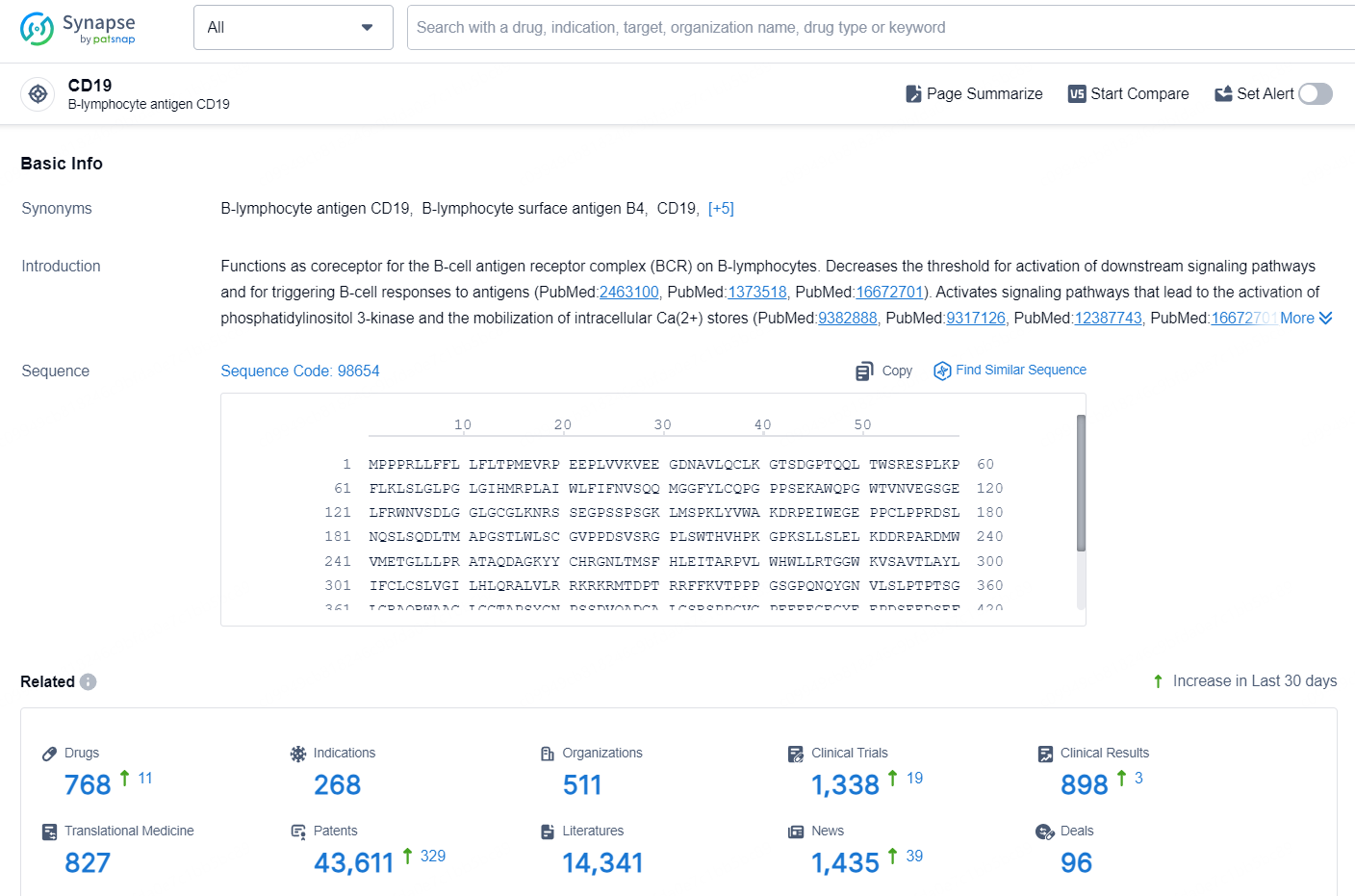
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 30, 2024, there are 768 investigational drug for the CD19 target, including 268 indications, 511 R&D institutions involved, with related clinical trials reaching 1338, and as many as 43611 patents.
CPTX-2309 is an autologous CAR-T and mRNA CAR-T drug developed by Capstan Therapeutics, Inc. The drug is designed to target CD19 and is primarily focused on treating autoimmune diseases within the immune system diseases therapeutic area. Currently, the drug is in the early Phase 1 of development.The autologous CAR-T and mRNA CAR-T drug types indicate that [CPTX-2309] is a form of cellular immunotherapy that involves the manipulation of a patient's own immune cells to target and combat specific diseases. By targeting the CD19 protein, the drug is intended to address immune system diseases, particularly autoimmune diseases, where the body's immune system mistakenly attacks its own cells.