Enanta Pharmaceuticals Announces Promising Phase 2a Results for EDP-323 in RSV-Challenged Healthy Adults
Enanta Pharmaceuticals, Inc. (NASDAQ:ENTA), a biotechnology company at the clinical-stage focused on developing leading small molecule drugs for virology and immunology, reported encouraging topline results from a Phase 2a human challenge study of EDP-323 in healthy adults infected with respiratory syncytial virus (RSV). The findings indicated that EDP-323 was generally safe, well-tolerated, and led to an 85-87% reduction in viral load area under the curve (AUC) as determined by qRT-PCR (p<0.0001), a 97-98% reduction in infectious viral load AUC via viral culture (p<0.0001), and a 66-78% reduction in total clinical symptoms score AUC (p<0.0001) compared to placebo. EDP-323, which has received Fast Track designation from the U.S. Food and Drug Administration (FDA), is a novel L-protein inhibitor currently being developed as a once-daily oral treatment for RSV.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"We are thrilled by the remarkable data that show a swift and sustained decrease in viral load. These findings rank among the most robust ever observed in an RSV challenge study, surpassing the high bar established by zelicapavir. The notable antiviral activity and symptom relief observed in this study underscore EDP-323’s potential as a safe, highly effective, direct-acting antiviral for RSV treatment," stated Scott T. Rottinghaus, M.D., Chief Medical Officer of Enanta Pharmaceuticals.
"The EDP-323 results represent significant progress towards our long-term objective of developing new treatments for respiratory infections like RSV, addressing the substantial need for safe and effective oral therapies. Enanta has an unparalleled portfolio of strong RSV replication inhibitors, with EDP-323, our L-protein inhibitor, and zelicapavir, our N-protein inhibitor, both in Phase 2 clinical trials. These different mechanisms could potentially be developed as once-daily monotherapies or in combination for specific patient groups," added Jay R. Luly, Ph.D., President and CEO of Enanta Pharmaceuticals.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
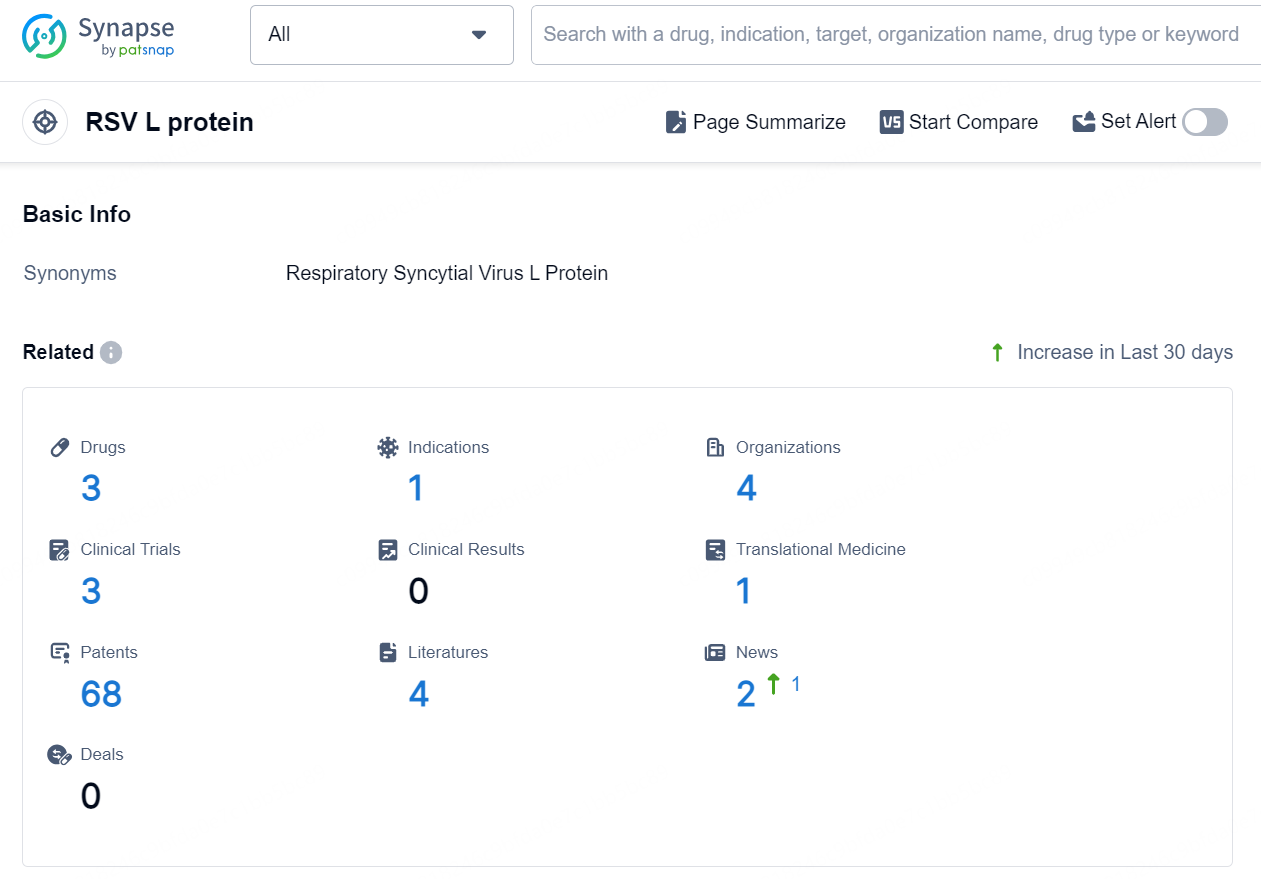
According to the data provided by the Synapse Database, As of September 30, 2024, there are 3 investigational drugs for the RSV L target, including 1 indication, 4 R&D institutions involved, with related clinical trials reaching 3, and as many as 68 patents.
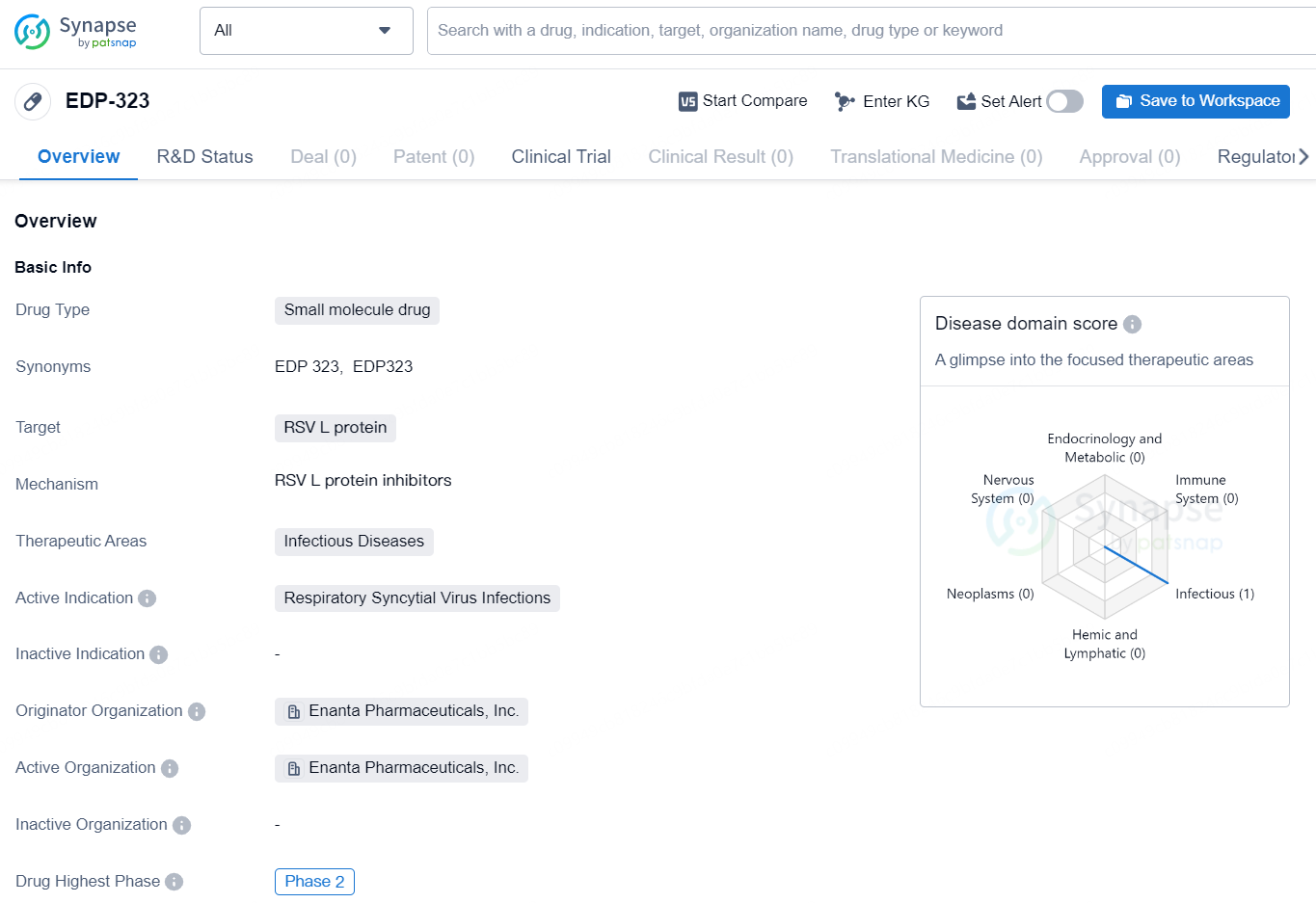
EDP-323 is a small molecule drug developed by Enanta Pharmaceuticals, Inc. It is specifically designed to target the RSV L protein and is being developed for the treatment of Respiratory Syncytial Virus (RSV) Infections, which falls under the therapeutic area of Infectious Diseases. As of now, EDP-323 has reached the highest phase of development, which is Phase 2, on a global scale.