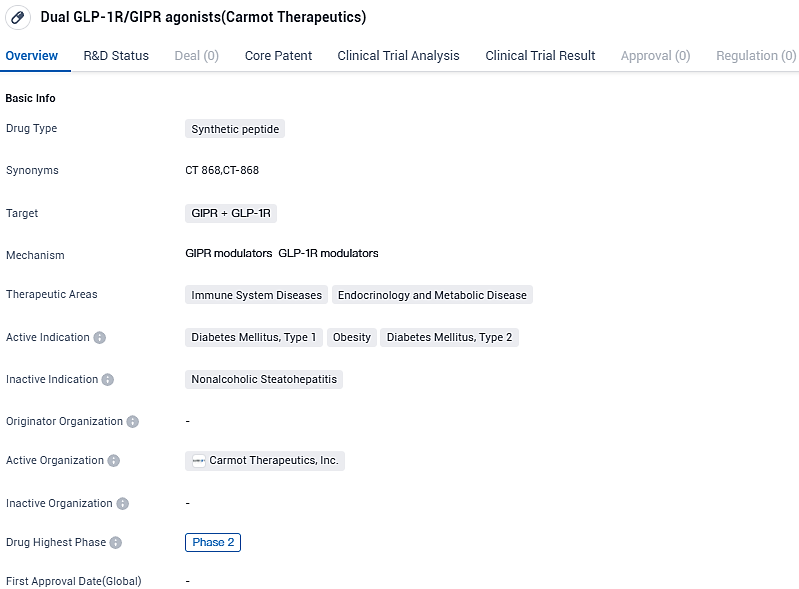
Carmot Therapeutics starts phase 2 trial for CT-868, a GLP-1/GIP agonist, to treat Type 1 Diabetes
A Phase 2 clinical trial for CT-868, a once-daily dual GLP-1/GIP receptor agonist, in adult subjects who are overweight or obese with T1D, has been initiated by Carmot Therapeutics Inc., a biotechnology company in the clinical phase committed to the creation of transformative treatments for metabolic diseases such as overweightness and diabetes.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Carmot previously showcased outcomes from a different Phase 2 clinical examination of CT-868. This revealed a placebo-controlled decrease in HbA1c by 2.31% from the starting point at the 26th week in participants with type 2 diabetes who were either overweight or obese.
The 4.0 mg dose was well-accepted, while the most recurring side effects were gastrointestinal and generally minor. The accumulated data, along with precedent experiments for preclinical and clinical action, further enforce the clinical and mechanistic reasoning to consider CT-868 as an additional therapeutic to insulin in treating T1D.
Beside this Phase 2 clinical testing announced, Carmot is also conducting an ongoing Phase 1b active comparator crossover testing to evaluate the impact of using CT-868 on glucose balance compared to liraglutide in T1D participants. "Advancing to the next level with this Phase 2 clinical examination is a significant progression in CT-868’s clinical course, and we are optimistic about probing CT-868's potential as a supplementary therapy for individuals managing with T1D," stated Manu Chakravarthy, MD, PhD, Chief Scientific & Medical Officer at Carmot.
"We are truly happy with the advancement in all our three clinical ventures and anticipate multiple data outcomes from our pipeline focused on obesity and diabetes by 2024." CT-868 is among the trio of clinical-phase product contenders in the treatment pipeline at Carmot that aim to tackle obesity and diabetes.
A crossover clinical trial for 24 T1D participants in Phase 1b will scope out the impact of CT-868, liraglutide, or placebo on glucose balance, measured through a mixed meal tolerance test in a manner independent of weight.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
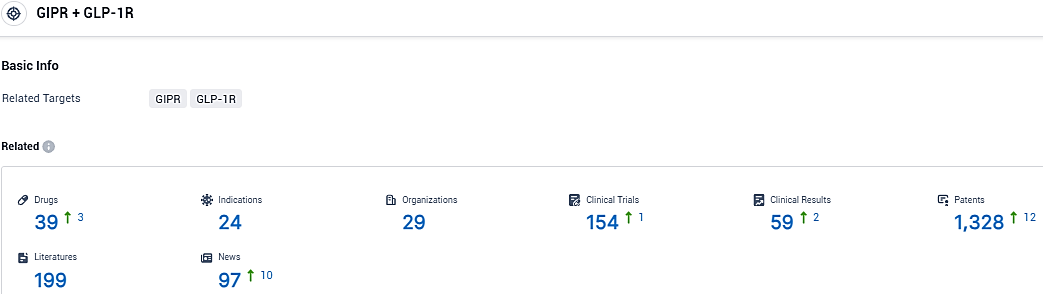
According to the data provided by the Synapse Database, As of November 22, 2023, there are 39 investigational drugs for the GIPR and GLP-1R target, including 24 indications, 29 R&D institutions involved, with related clinical trials reaching 154, and as many as 1328 patents.
CT-868 is a once-daily subcutaneous injectable, dual GLP-1/GIP receptor agonist being developed as an adjunct to insulin for the treatment of people with type 1 diabetes with overweight or obesity. It is currently being studied in a Phase 2 proof-of-concept clinical trial in people with overweight or obesity with T1D and an active comparator Phase 1b mechanism of action crossover clinical trial to assess glucose homeostasis in people with T1D.