SNS-101(Sensei Biotherapeutics): A Quick Look at Its R&D Progress and Clinical Results from the 2023 SITC
The latest clinical result of SNS-101(Sensei Biotherapeutics) in patients with advanced solid tumors was reported at the 2023 SITC Congress, demenstrating its potential efficacy and paving the way for future research.
SNS-101(Sensei Biotherapeutics)'s R&D Progress
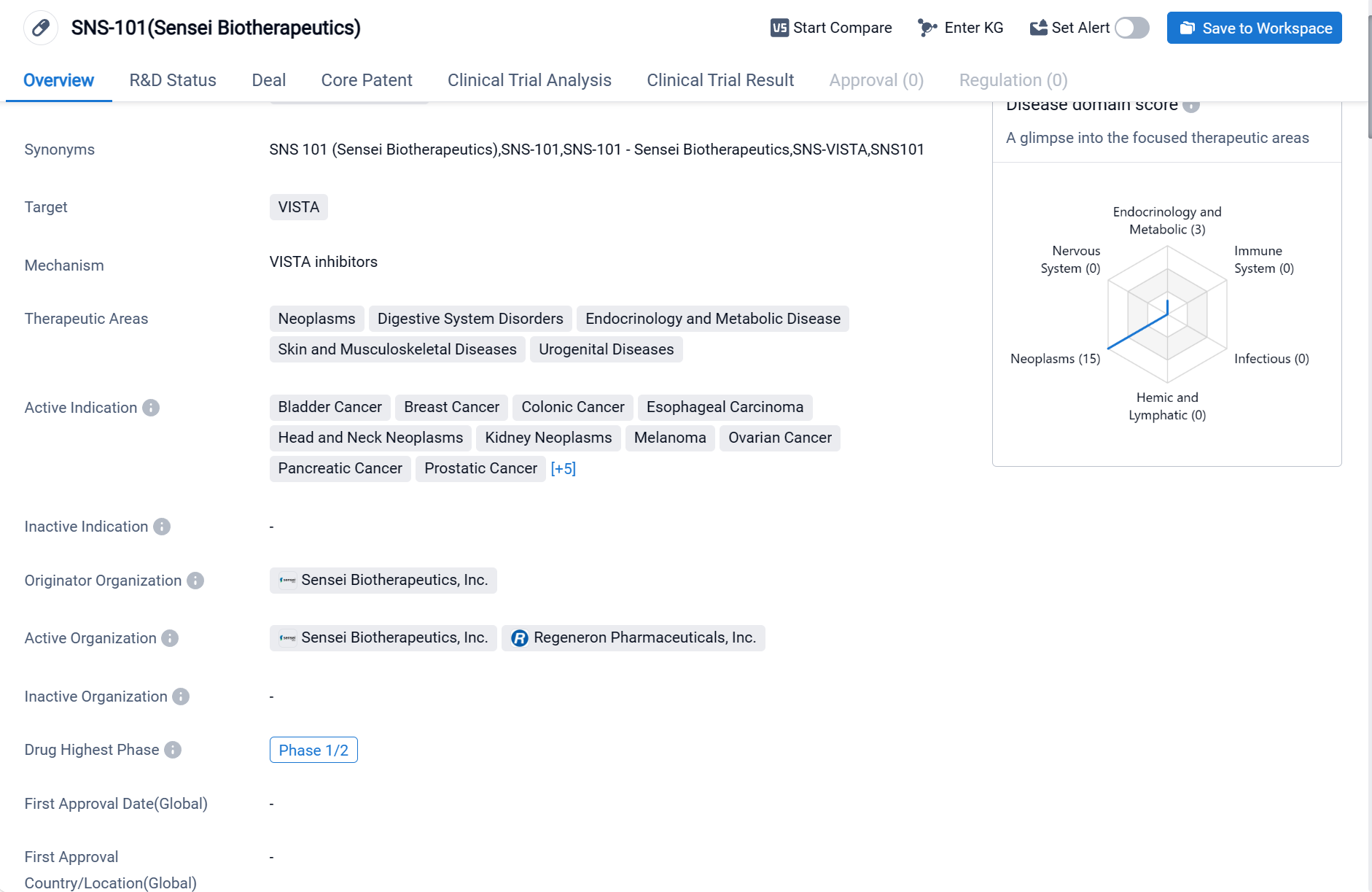
SNS-101, developed by Sensei Biotherapeutics, is a monoclonal antibody drug that targets VISTA. The drug has shown potential in treating various therapeutic areas, including neoplasms, digestive system disorders, endocrinology and metabolic diseases, skin and musculoskeletal diseases, and urogenital diseases.
According to the Patsnap Synapse, SNS-101(Sensei Biotherapeutics) is currently in the highest phase of clinical development, Phase 1/2, globally. And the clinical trial areas for SNS-101(Sensei Biotherapeutics) are primarily in the United States. The key indication is Sarcoma. 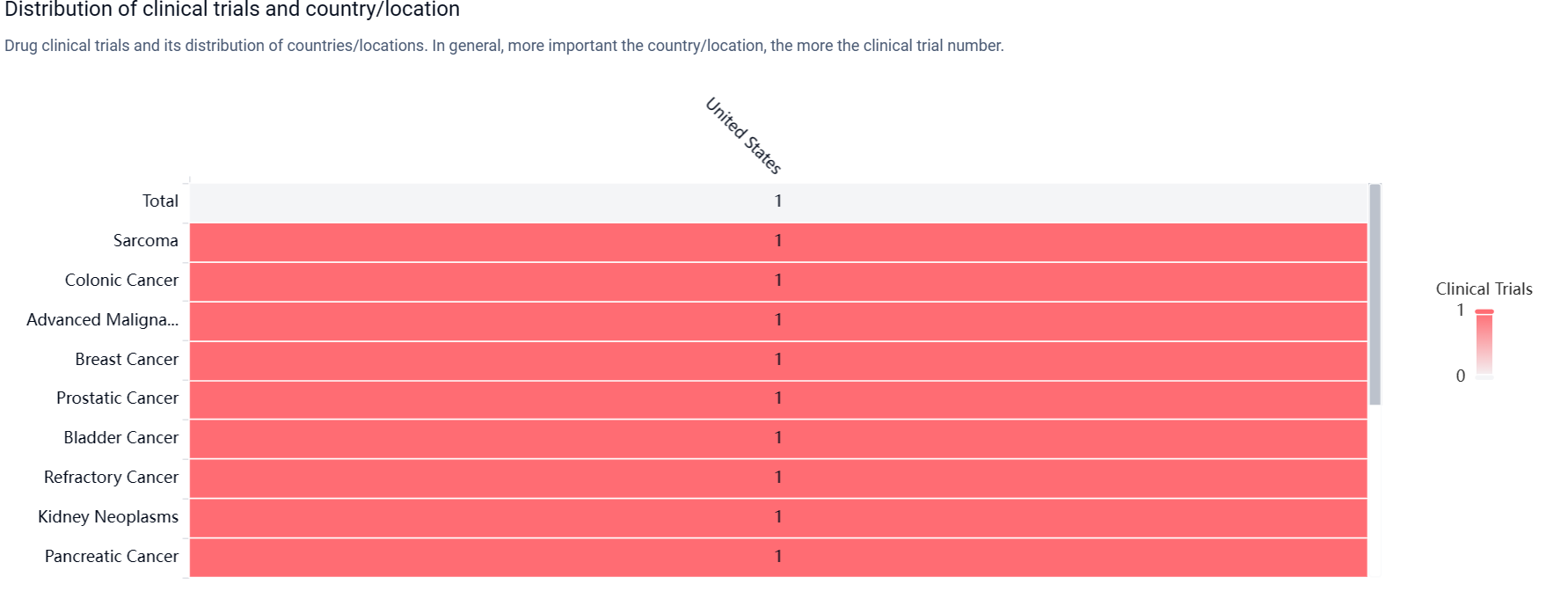
Detailed Clinical Result of SNS-101(Sensei Biotherapeutics)
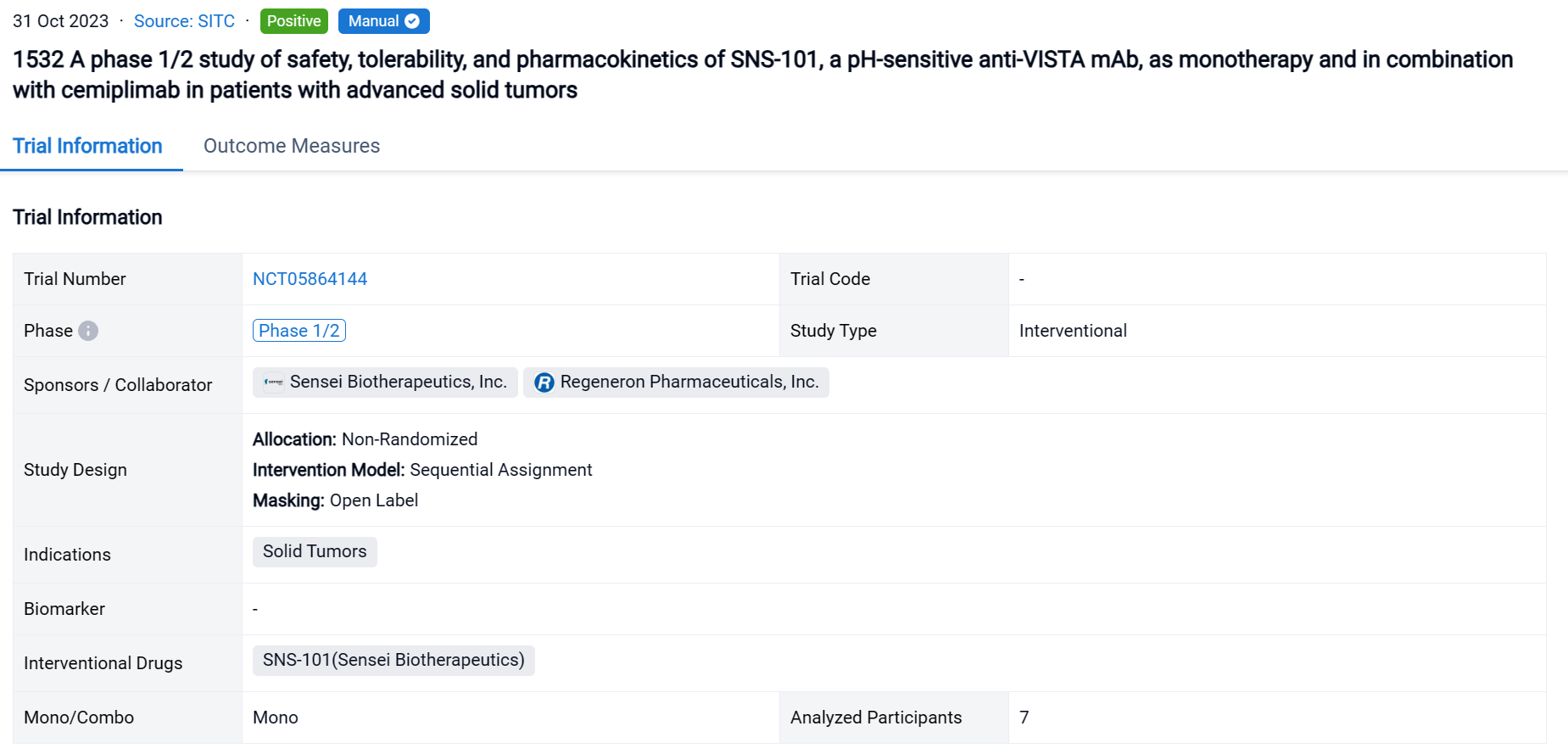
This is a first-in-human, open-label, multi-center, dose escalation and expansion study to evaluate the safety, tolerability, pharmacokinetics (PK), pharmacodynamics and efficacy of SNS-101 as monotherapy or in combination with cemiplimab in patients with advanced solid tumors (NCT05864144).
In this study, patients with advanced solid tumors (NCT05864144). The study is being conducted in 3 parts: Part A: Phase 1 (P1) Monotherapy Dose Escalation (SNS-101 alone); Part B: P1 Combination Dose Escalation (SNS-101 + cemiplimab); Part C: Phase 2 (P2) Expansion Cohorts (SNS-101 ± cemiplimab). Patients will receive SNS-101 ± cemiplimab as intravenous infusion(s) every 3 weeks and may continue until confirmed progressive disease or unacceptable toxicity. Dose escalation follows the Bayesian Optimal Interval Design until the Maximum Tolerated Dose (MTD)/Recommended Phase 2 Dose (RP2D) is determined. Primary objectives include safety, tolerability and RP2D/MTD (P1), and evaluation of anti-tumor activity (P2). Safety and tolerability assessments include monitoring of dose limiting toxicities (DLTs) and adverse events (AEs), PK, anti-drug antibodies and inflammatory cytokine release. Tumor imaging and T-cell immunophenotyping are being utilized to monitor responses.
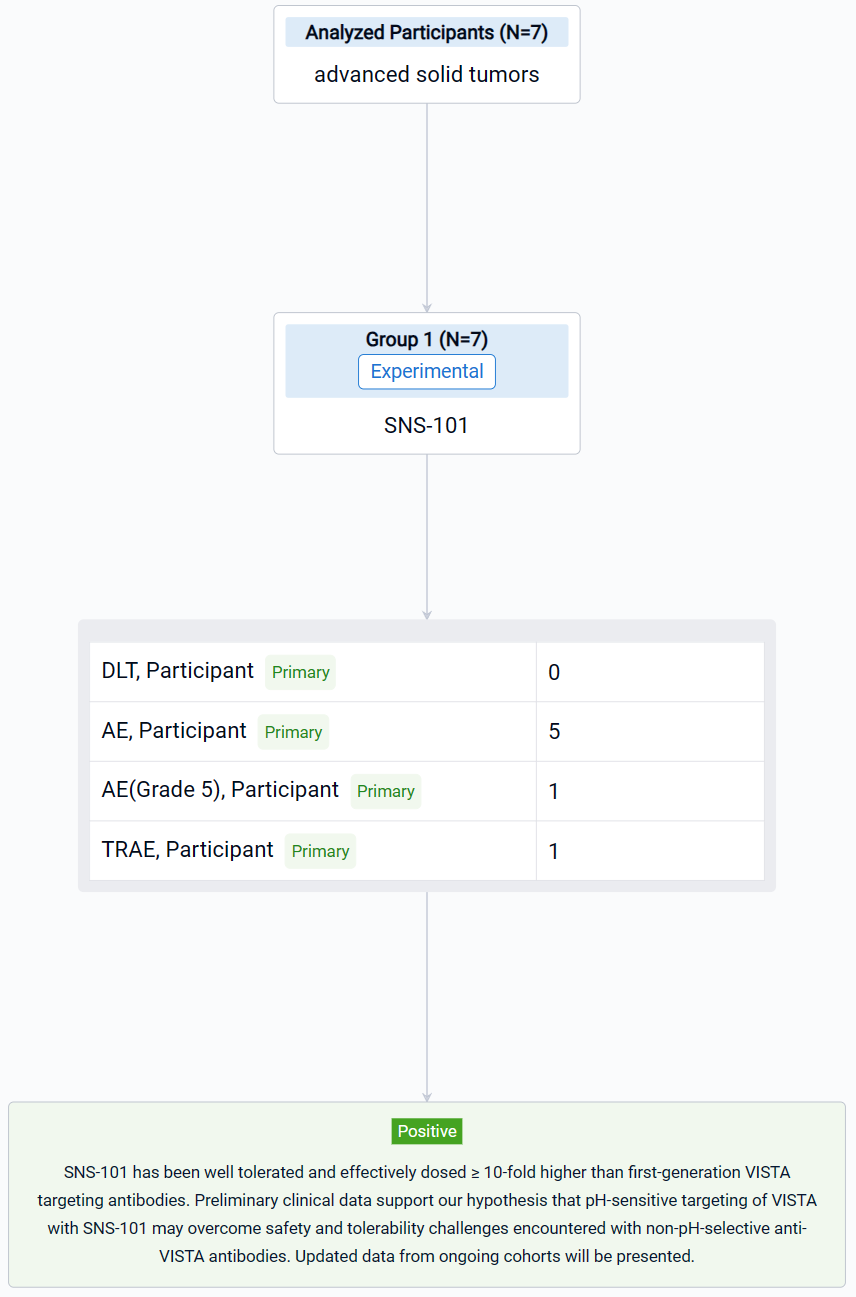
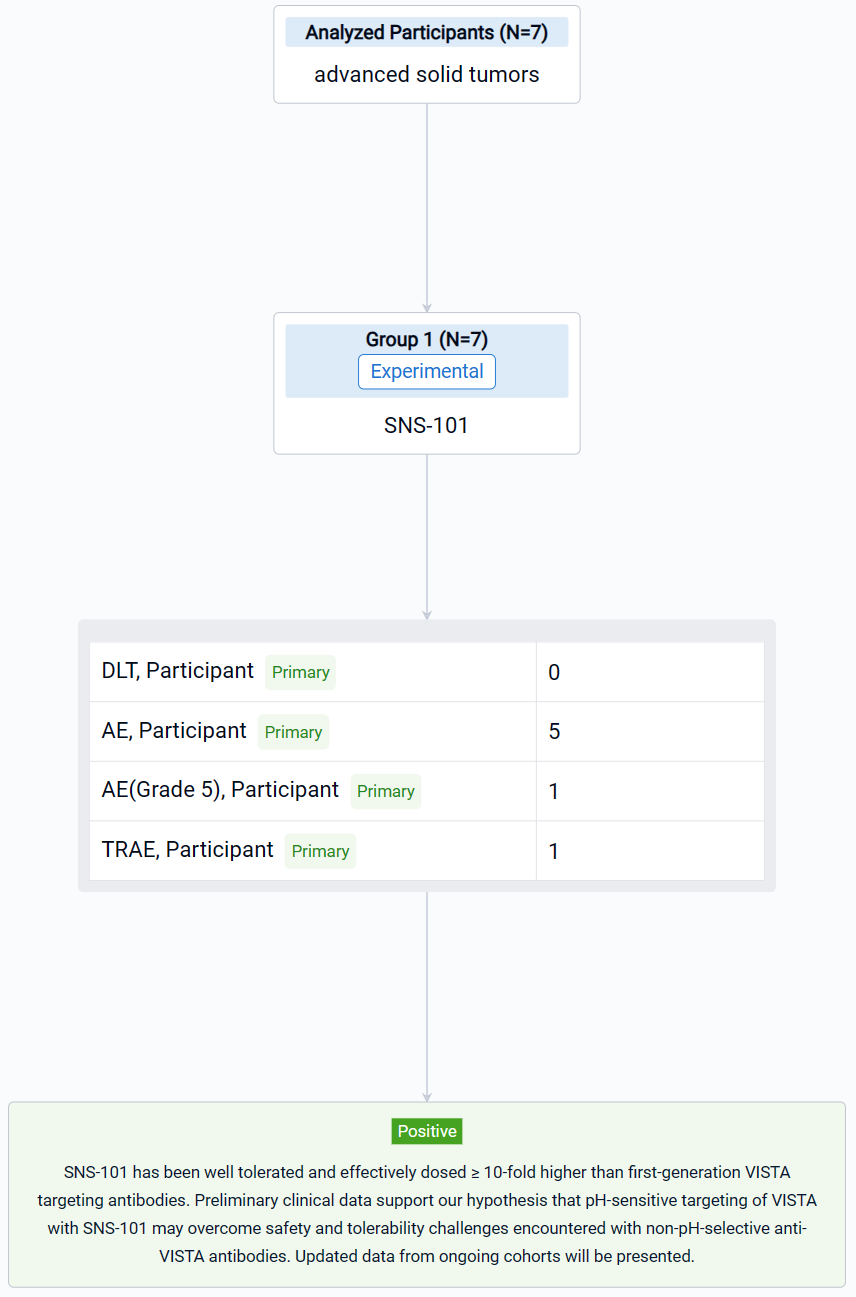
The result showed that as of August 31, 2023, 7 patients were enrolled in Part A across three dosing cohorts (0.3 mg/kg, 1 mg/kg and 3 mg/kg). No DLTs or CRS events were noted. Nine AEs (8/9 Grade 1–2) have been reported in 5 patients. One Grade 5 AE related to disease progression and not to the treatment was observed. One AE, dermatitis acneiform, is considered treatment-related. Infusions have not required premedications. PK results show high concordance with preclinical modeling data, demonstrating dose-proportional exposure, linear elimination kinetics, and suggesting the absence of TMDD.
It can be concluded that SNS-101 has been well tolerated and effectively dosed ≥ 10-fold higher than first-generation VISTA targeting antibodies. Preliminary clinical data support our hypothesis that pH-sensitive targeting of VISTA with SNS-101 may overcome safety and tolerability challenges encountered with non-pH-selective anti-VISTA antibodies. Updated data from ongoing cohorts will be presented.
How to Easily View the Clinical Results Using Synapse Database?
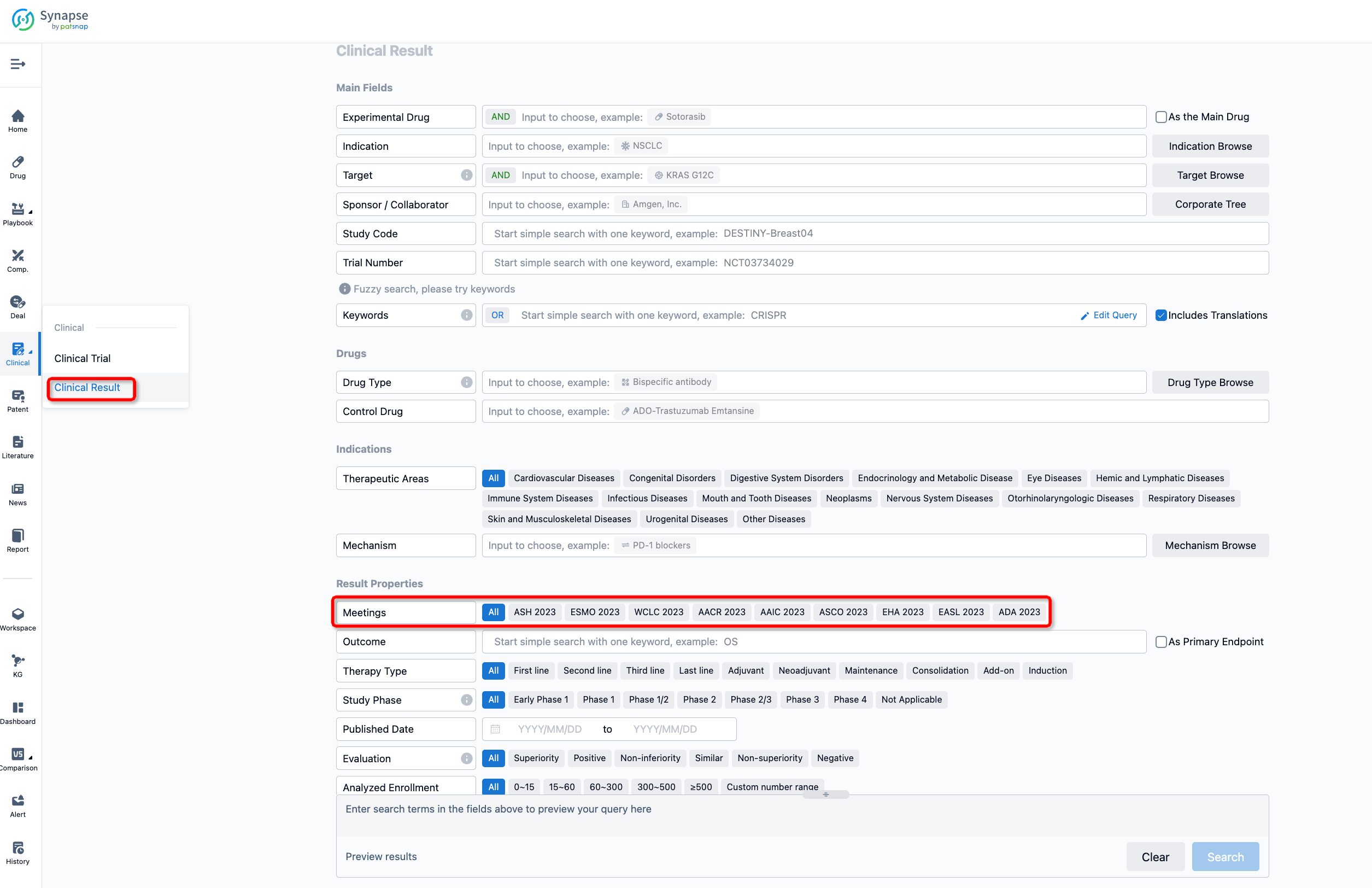
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
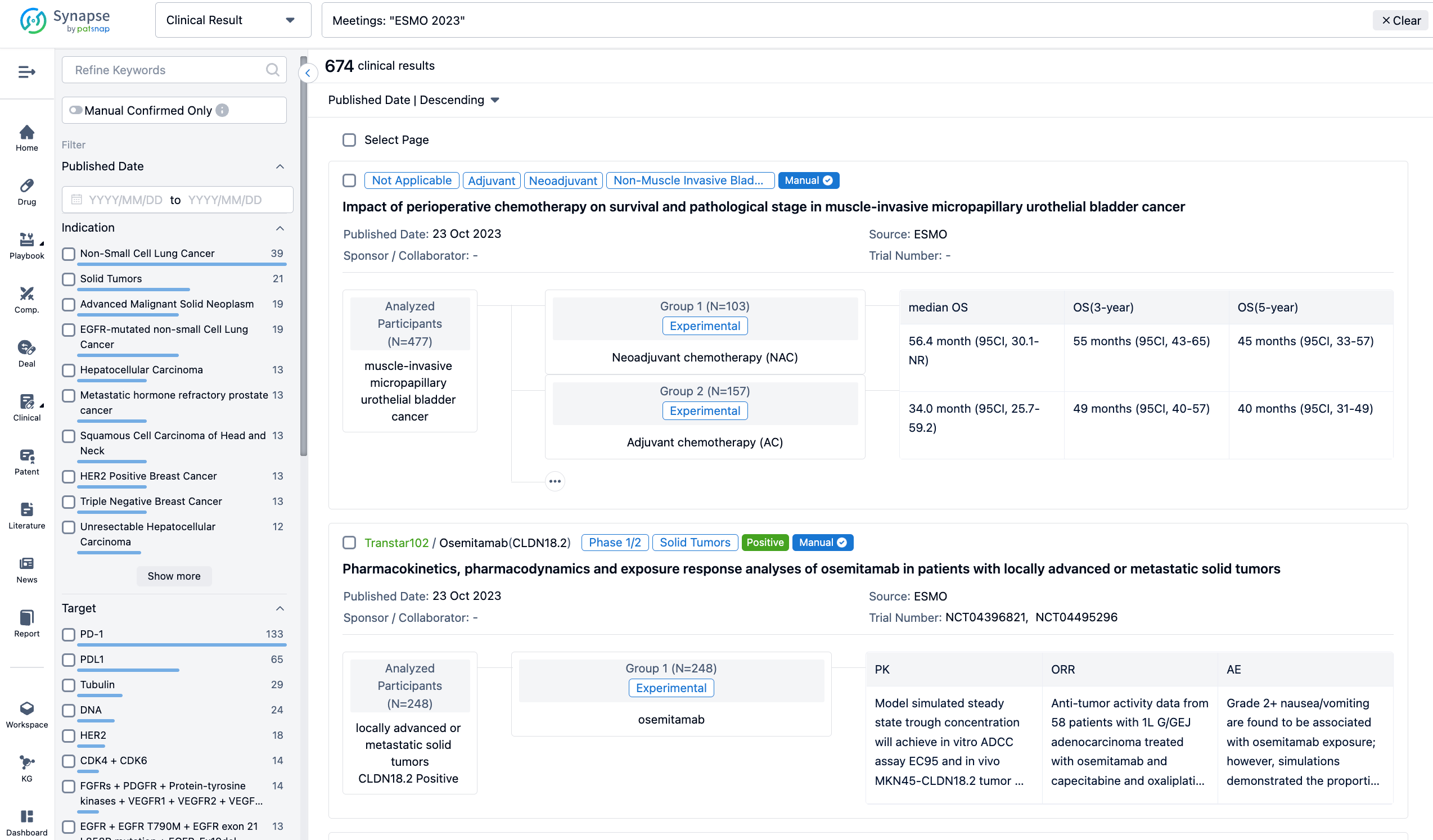
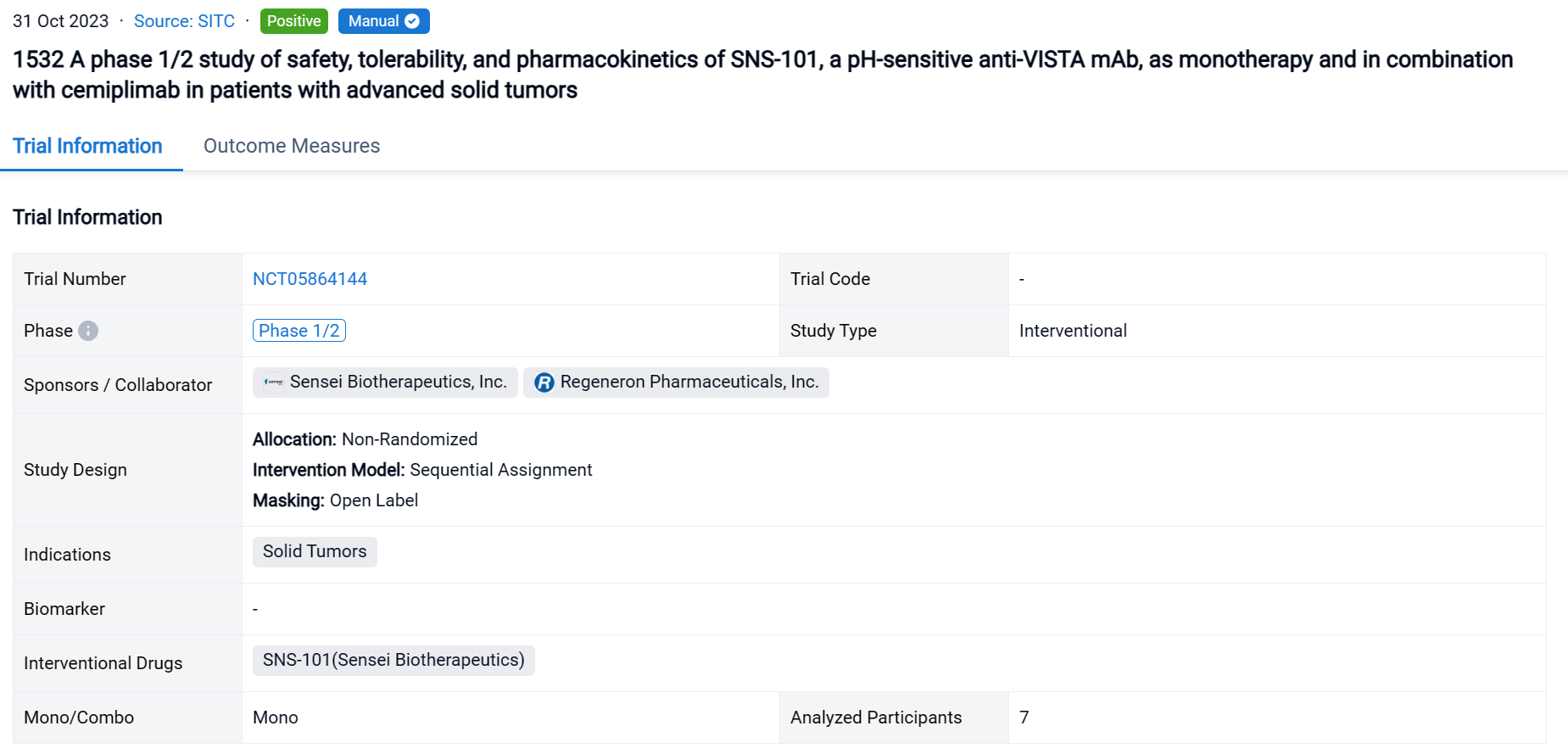
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
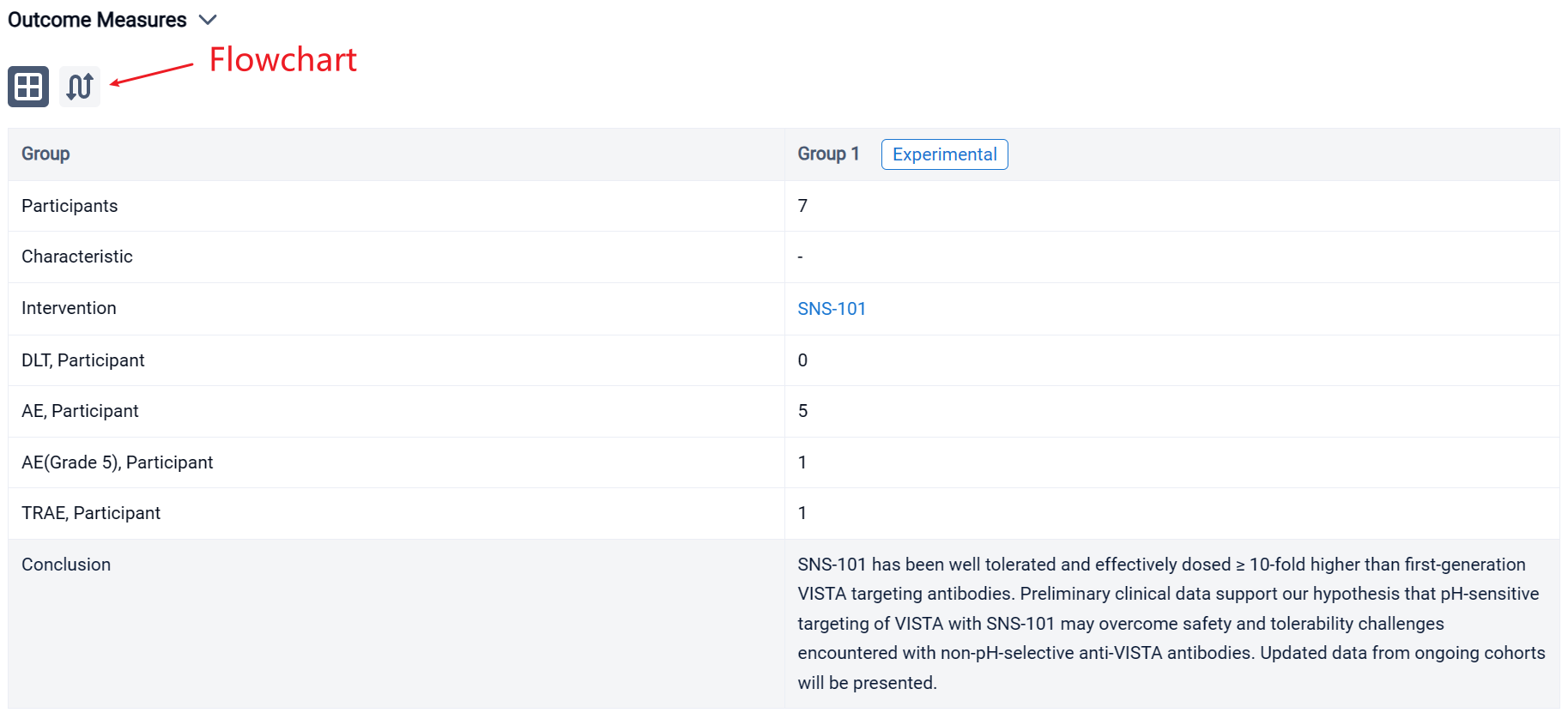
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
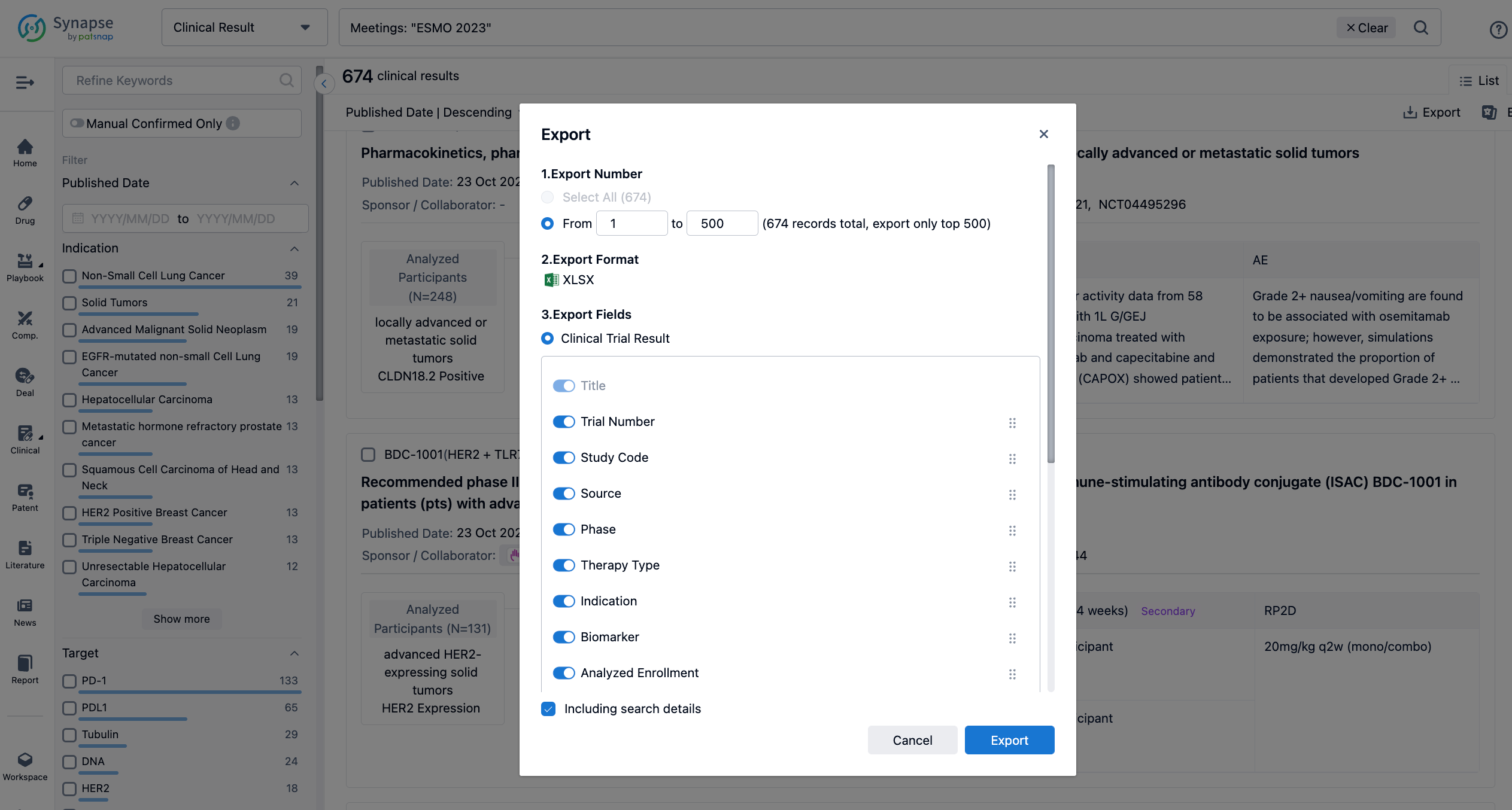
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!