What are CTLA4 inhibitors and how do you quickly get the latest development progress?
CTLA-4 (Cytotoxic T-lymphocyte-associated protein 4) is a crucial immune checkpoint receptor found on the surface of T cells. It plays a vital role in regulating the immune response by downregulating T cell activation. CTLA-4 acts as a negative regulator, preventing excessive immune activation and maintaining immune homeostasis. By binding to its ligands, CD80 and CD86, on antigen-presenting cells, CTLA-4 inhibits T cell activation and proliferation, thus suppressing immune responses. Understanding the role of CTLA-4 has led to the development of immunotherapies, such as immune checkpoint inhibitors, which block CTLA-4 to enhance anti-tumor immune responses in cancer patients.
CTLA-4 has further enhanced clinicians' understanding of immunotherapy, increasing anticipation for dual immunotherapies. Currently, the only CTLA-4 inhibitors approved by the U.S. FDA are Ipilimumab and Tremelimumab. Ipilimumab has only been approved for monotherapy in specific cases of melanoma, with all others being combination therapies. There are still issues, such as limited types of drugs, narrow indication range, lack of optimal dose and treatment strategies. As of now, there are a total of 238 CTLA-4 clinical trials registered on the ClinicalTrails.gov website, of which 119 are ongoing. The CTLA-4 clinical trials registered and conducted at the China Clinical Trial Registry are all combination therapies, though not many. Besides, the existing combined therapies have limited effectiveness despite various attempts and explorations.
In conclusion, due to the complexity, uncertainty and certain risks associated with immunotherapy, more comprehensive evidence-based medical evidence is still needed. We should accurately select advantageous populations for immunotherapy through precise biomarkers, and precisely predict the efficacy and risk of immunotherapy. The combined use of CTLA-4 inhibitors at different tumor types and treatment stages should be conducted prudently under the guidance of evidence-based medicine and relevant domestic and foreign guidelines.
The mechanism of action of CTLA-4 inhibitors
From a biomedical perspective, CTLA4 inhibitors are a type of medication that target the protein called cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). This protein is found on the surface of T cells, which are a type of white blood cell involved in the immune response. By inhibiting CTLA-4, these inhibitors can enhance the immune system's ability to attack cancer cells.
CTLA4 inhibitors, such as ipilimumab, work by blocking the interaction between CTLA-4 and its binding partners, which normally downregulate the immune response. By blocking this interaction, CTLA4 inhibitors prevent the suppression of T cell activity, allowing them to mount a stronger attack against cancer cells.
These inhibitors have been approved for the treatment of certain types of cancer, including melanoma, a type of skin cancer. They are often used in combination with other cancer treatments, such as chemotherapy or targeted therapy, to improve treatment outcomes. However, it's important to note that CTLA4 inhibitors can also cause immune-related side effects, as they can lead to an overactive immune response that may affect healthy tissues and organs. Therefore, their use requires careful monitoring and management of potential side effects.
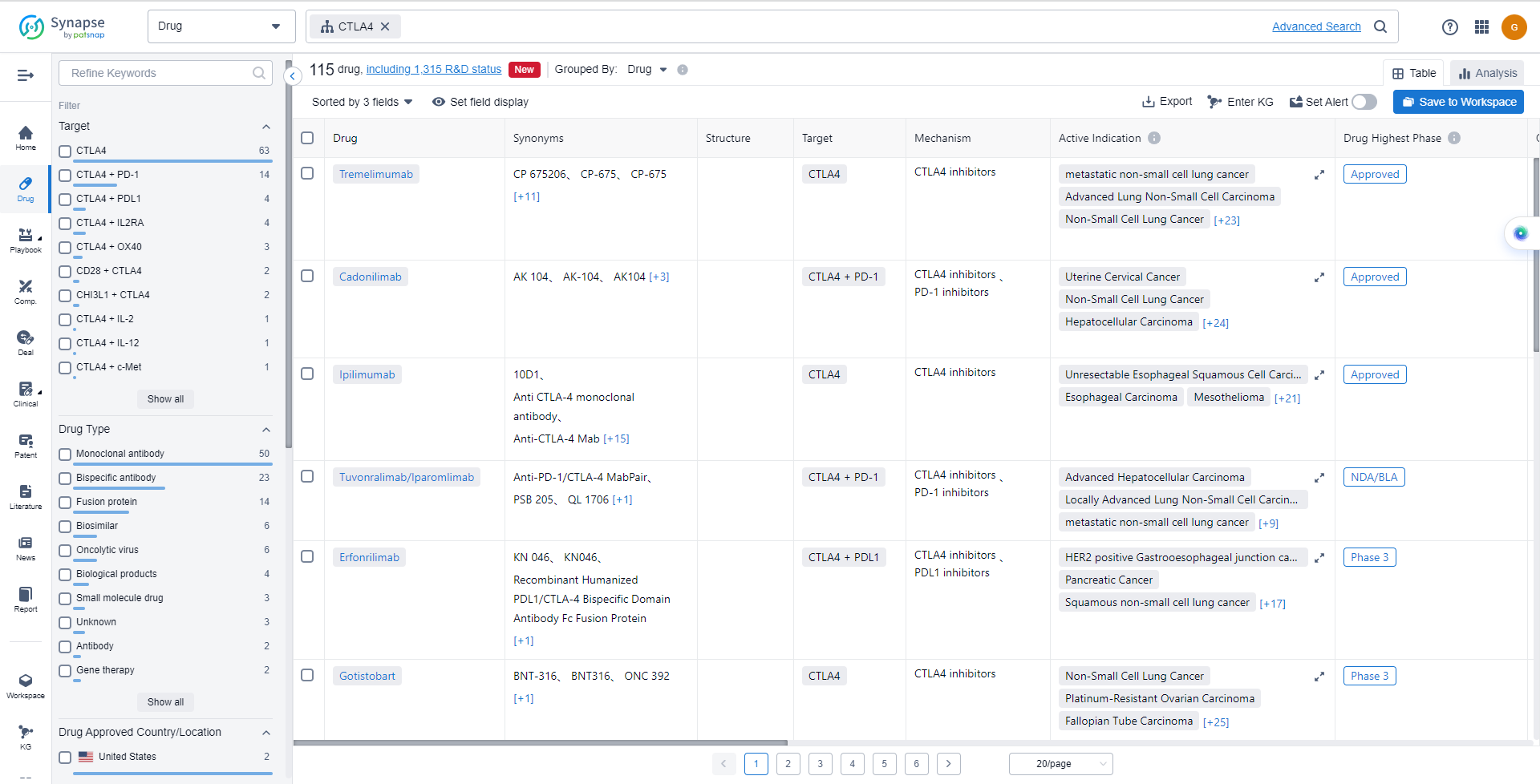
List of CTLA4 Inhibitors
The currently marketed CTLA4 inhibitors include:
- Tremelimumab
- Cadonilimab
- Ipilimumab
- Tuvonralimab/Iparomlimab
- Erfonrilimab
- Gotistobart
- Pembrolizumab/Quavonlimab
- Prolgolimab/nurulimab
- Quavonlimab
- Volrustomig
For more information, please click on the image below.
What are CTLA4 inhibitors used for?
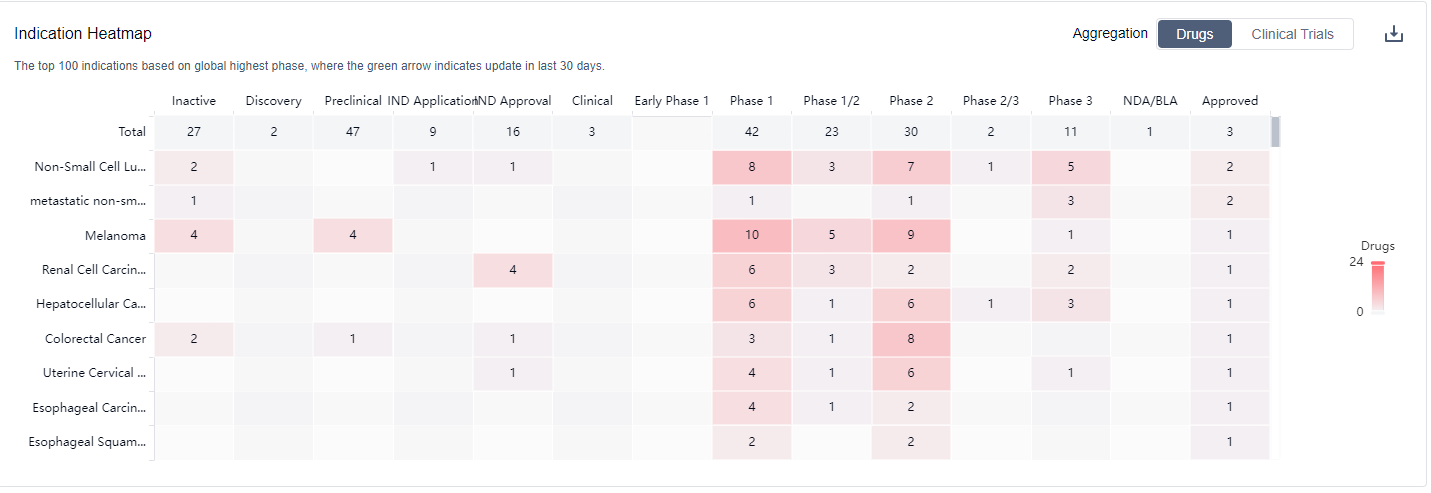
CTLA4 inhibitors are used for the treatment of solid tumors, particularly advanced solid tumors, including melanoma, especially stage IV melanoma, hepatocellular carcinoma, metastatic non-small cell lung cancer, non-small cell lung cancer, metastatic colorectal cancer, metastatic breast cancer, metastatic renal cell carcinoma, metastatic gastric cancer, squamous cell carcinoma, and transitional cell carcinoma. For more information, please click on the image below to log in and search.
How can you get the most recent advancements in CTLA4 inhibitors?
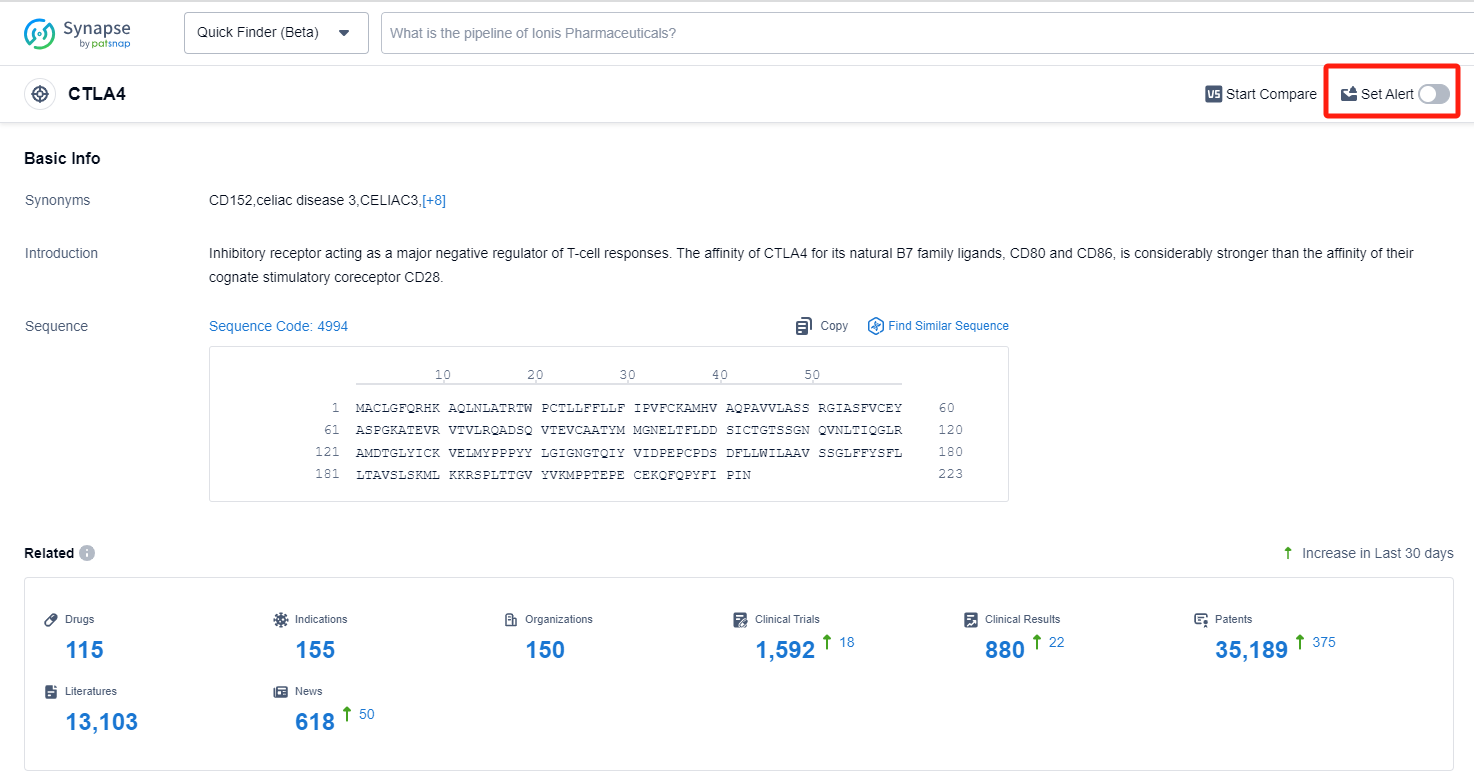
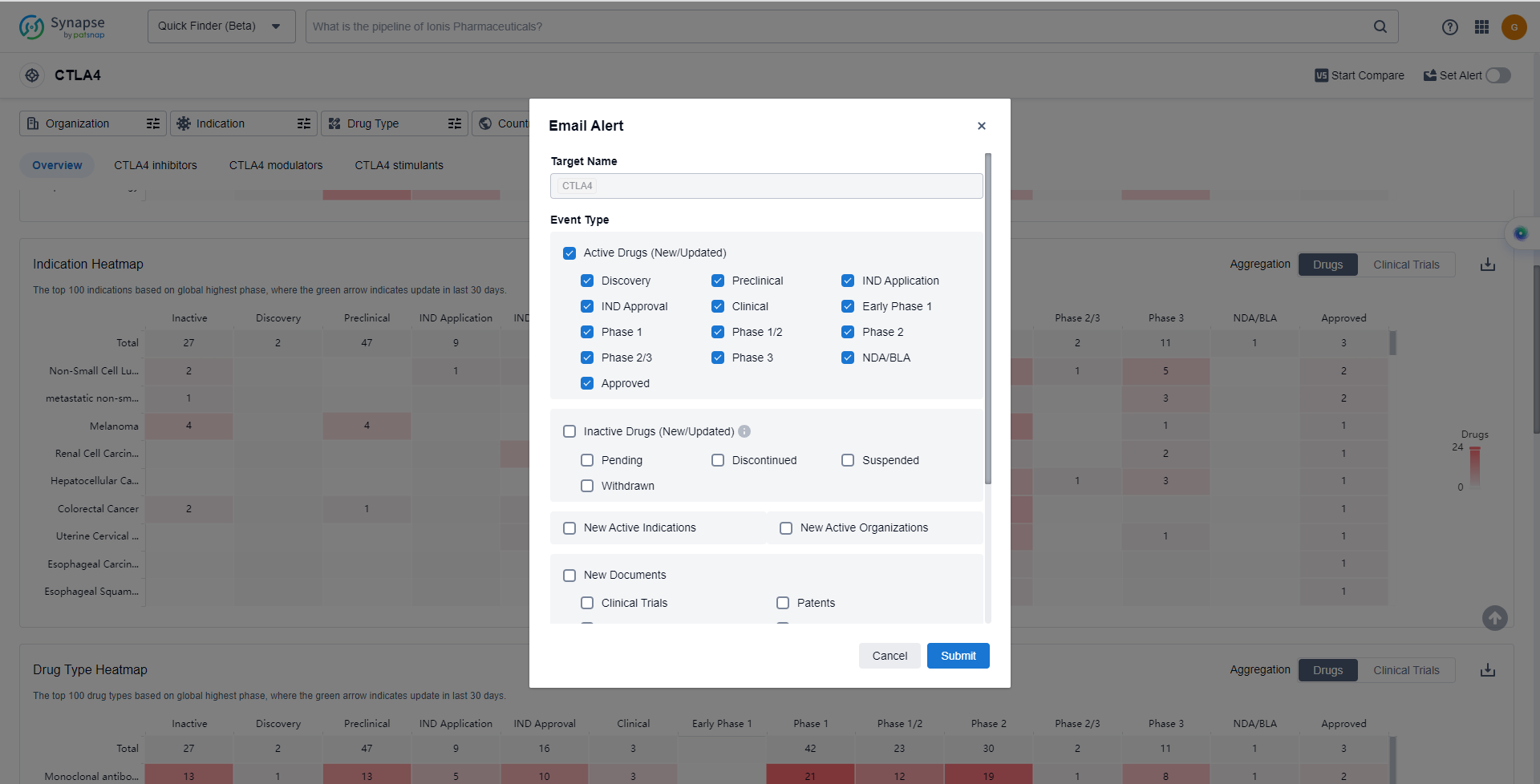
In the Synapse database, you can keep abreast of the latest research and development advances of CTLA4 inhibitors anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!