Casdozokitug: A Quick Look at Its R&D Progress and Clinical Results from the 2024 ASCO_GI
On 18 Jan 2024, the latest clinical results from a phase 2 study of triplet blockade of the IL-27, PD-(L)1, and VEGF pathways with casdozokitug in combination with atezolizumab (atezo) and bevacizumab (bev) in patients with unresectable, locally advanced, or metastatic hepatocellular carcinoma (uHCC) were presented in 2024 ASCO_GI.
Casdozokitug's R&D Progress
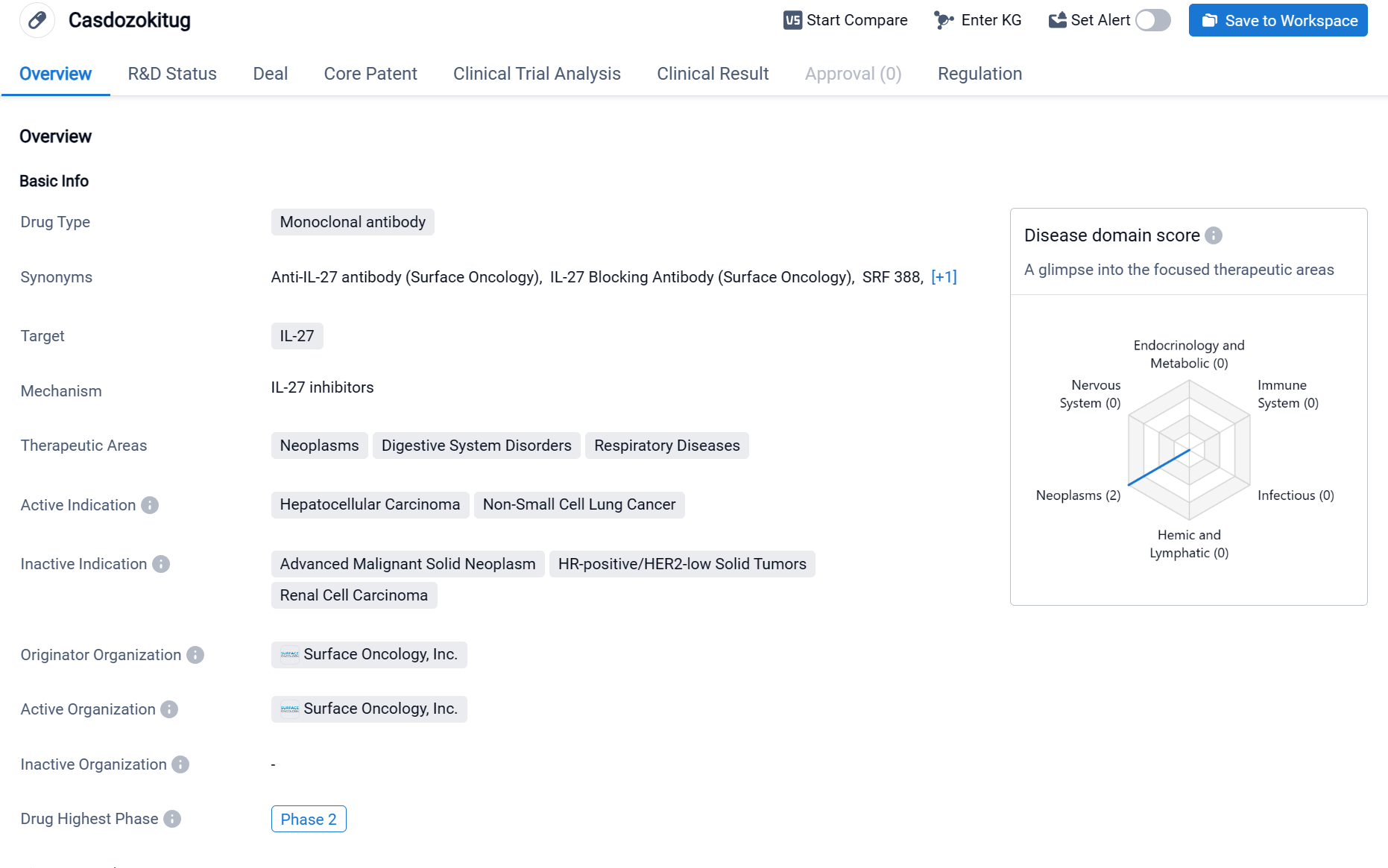
Casdozokitug is a monoclonal antibody drug that targets IL-27, a protein involved in immune regulation. The drug has shown potential in treating neoplasms, digestive system disorders, and respiratory diseases. Specifically, it has demonstrated efficacy in treating hepatocellular carcinoma and non-small cell lung cancer, two types of cancer that are known to be difficult to treat.
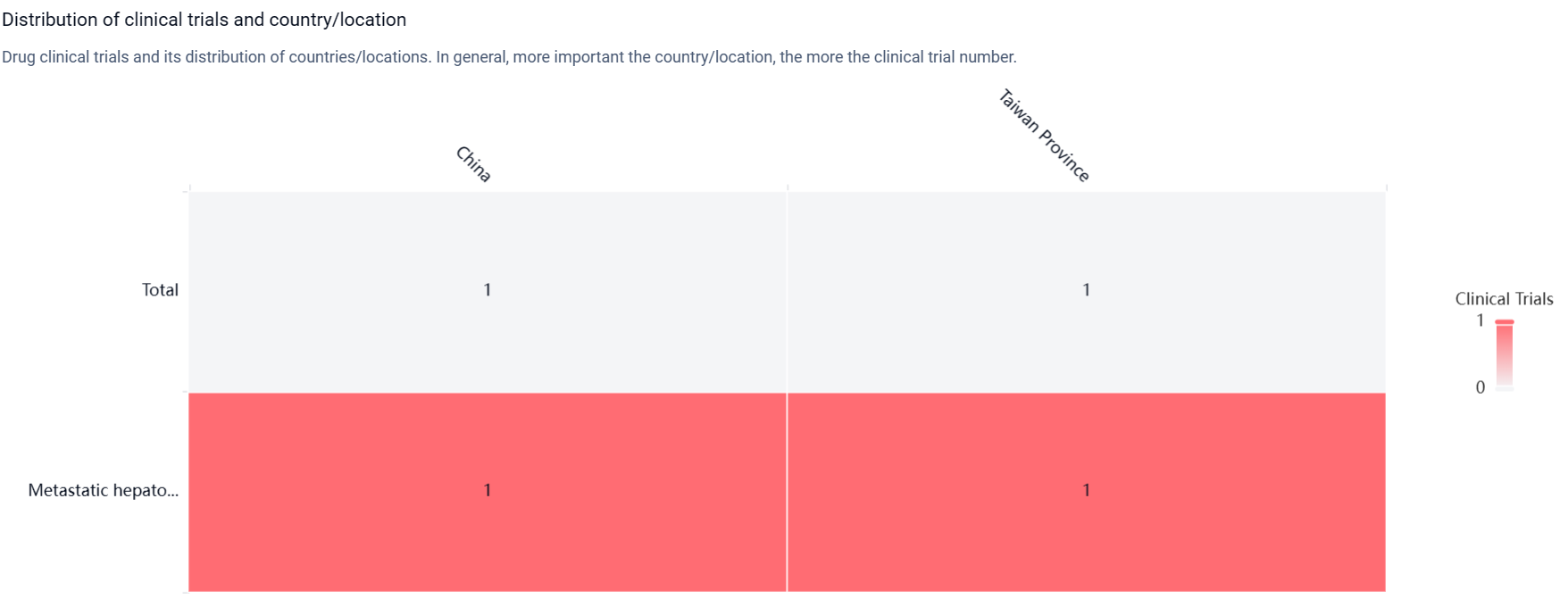
According to the Patsnap Synapse, Casdozokitug is being developed by Surface Oncology, Inc. and is currently in Phase 2 of clinical trials, which is the highest phase of development globally. And the clinical trial distributions for Casdozokitug are primarily in China and Taiwan Province. The key indication is Metastatic hepatocellular carcinoma.
Detailed Clinical Result of Casdozokitug
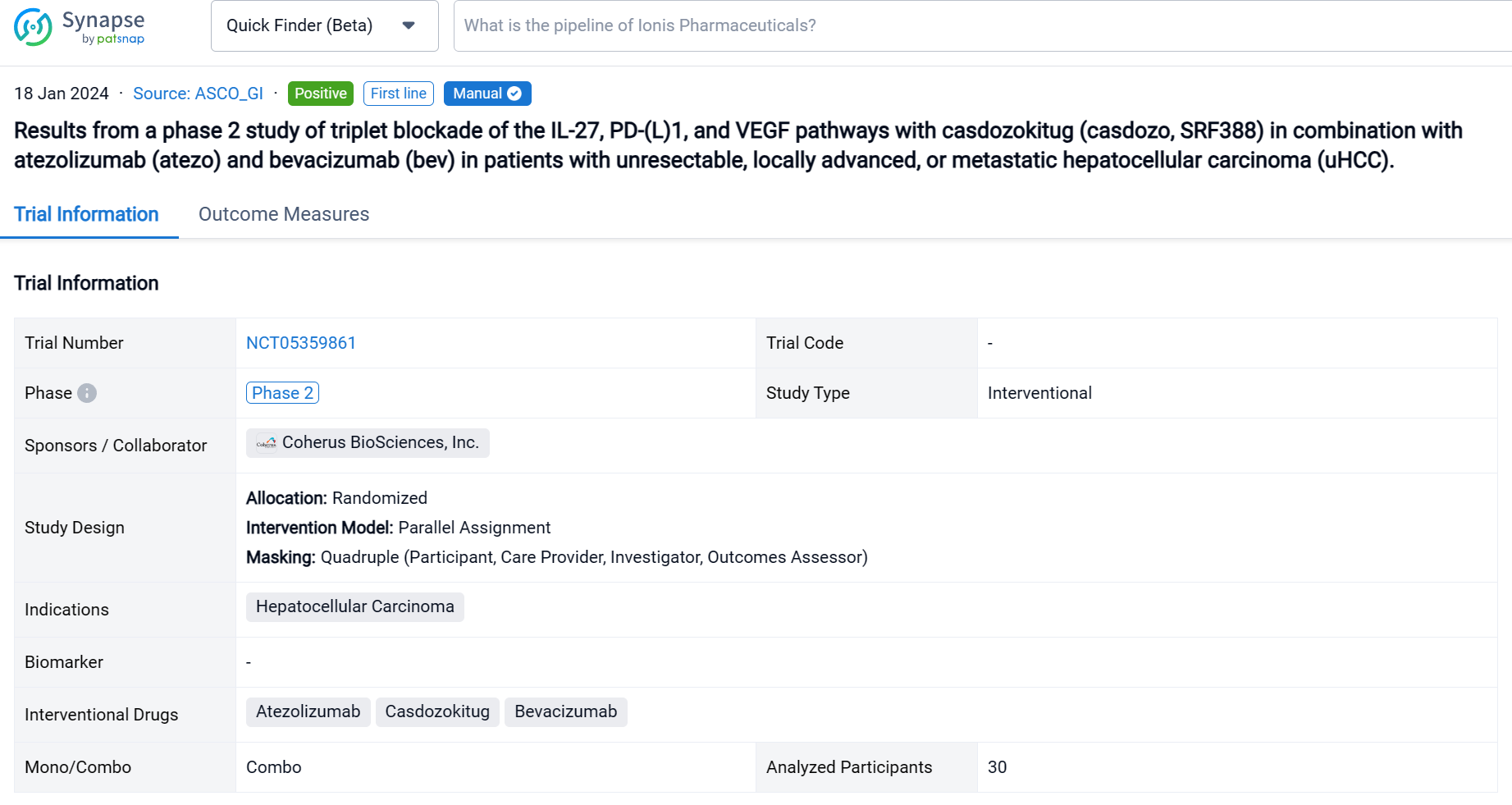
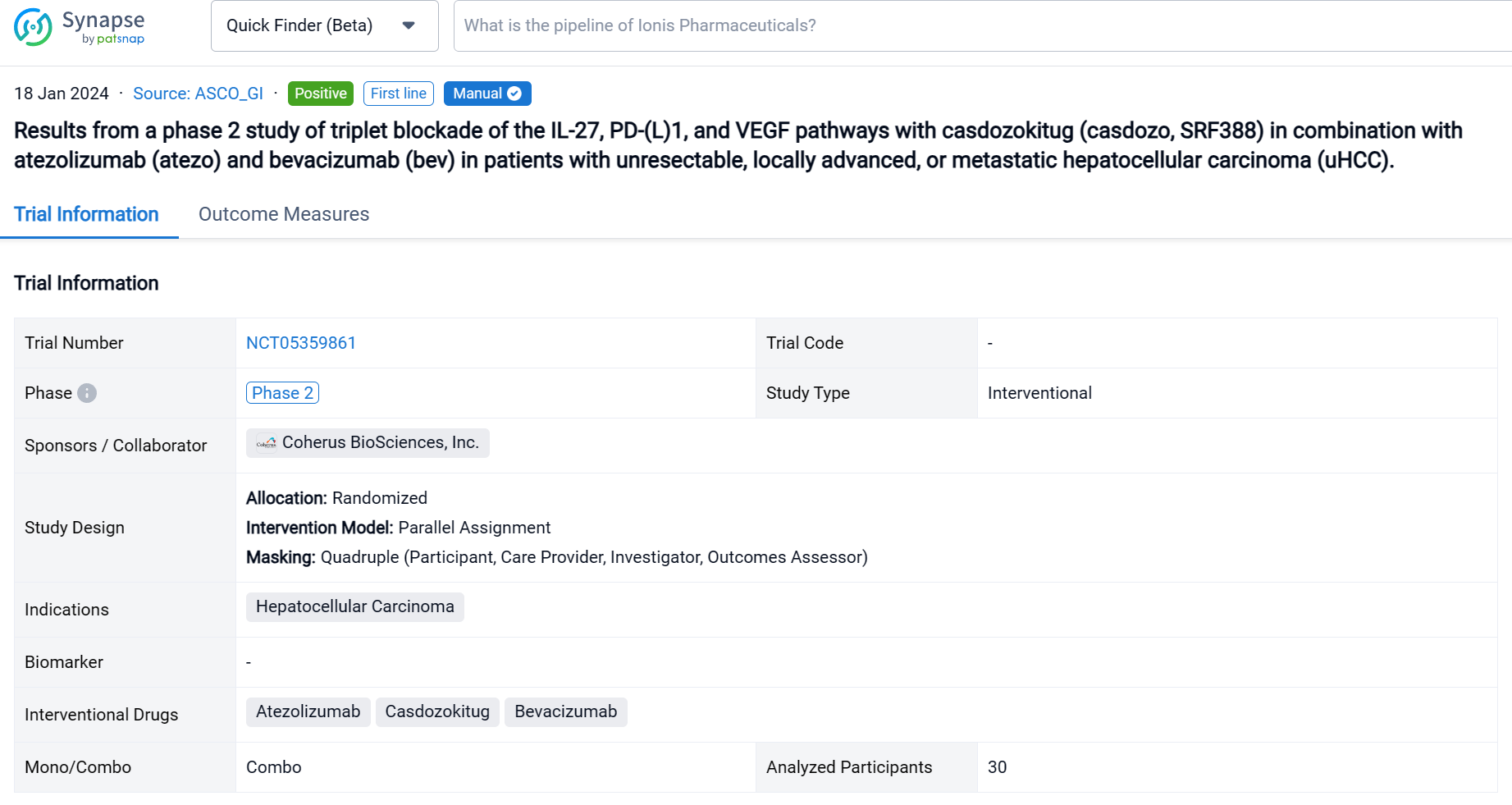
This randomized, parallel assignment, quadruple blind clinical trial (NCT05359861) was aimed to assess the safety and antitumor activity of Casdozokitug combination with atezo/bev in uHCC.
In this study, patients (pts) with untreated uHCC received casdozo 10 mg/kg, atezo 1200 mg, and bev 15mg/kg IV every 3 weeks. Primary endpoints were safety and tolerability, with key secondary endpoints being investigator-assessed ORR by RECIST1.1 and mRECIST.
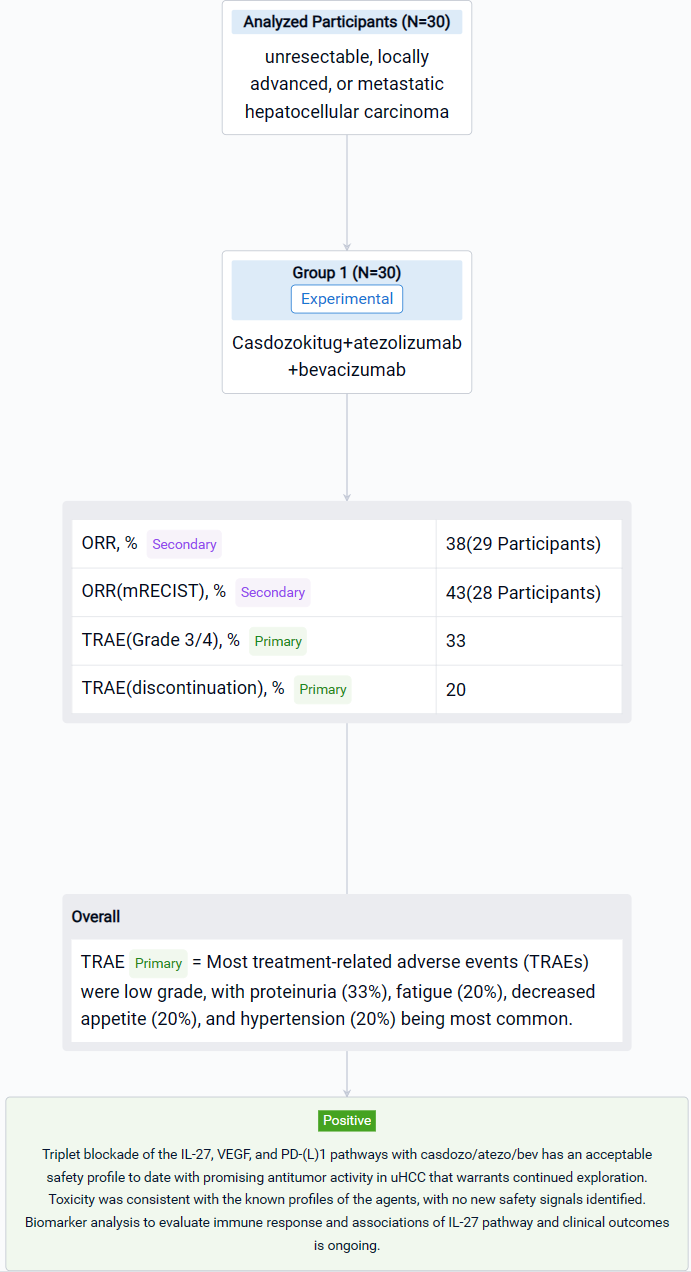
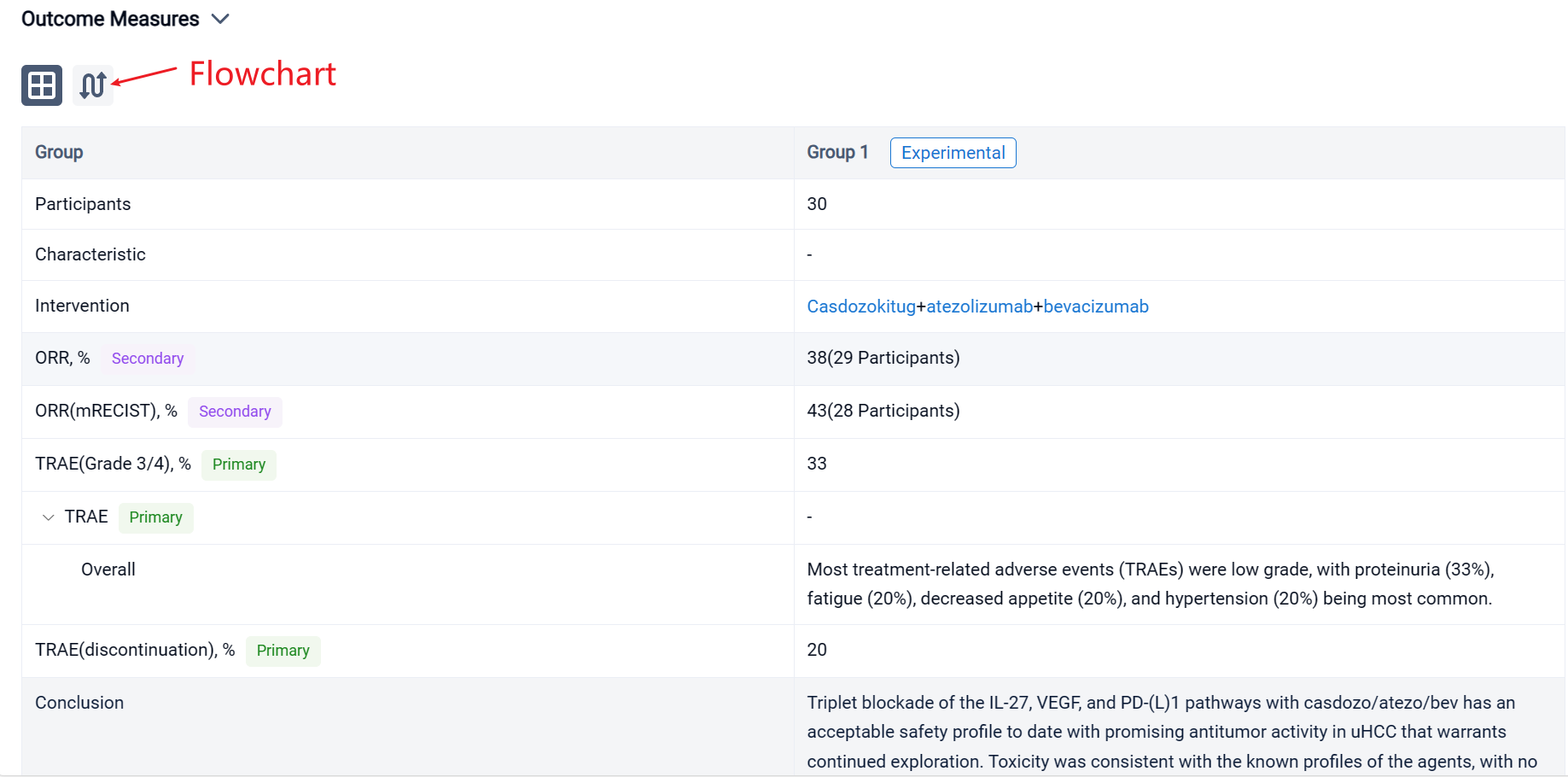
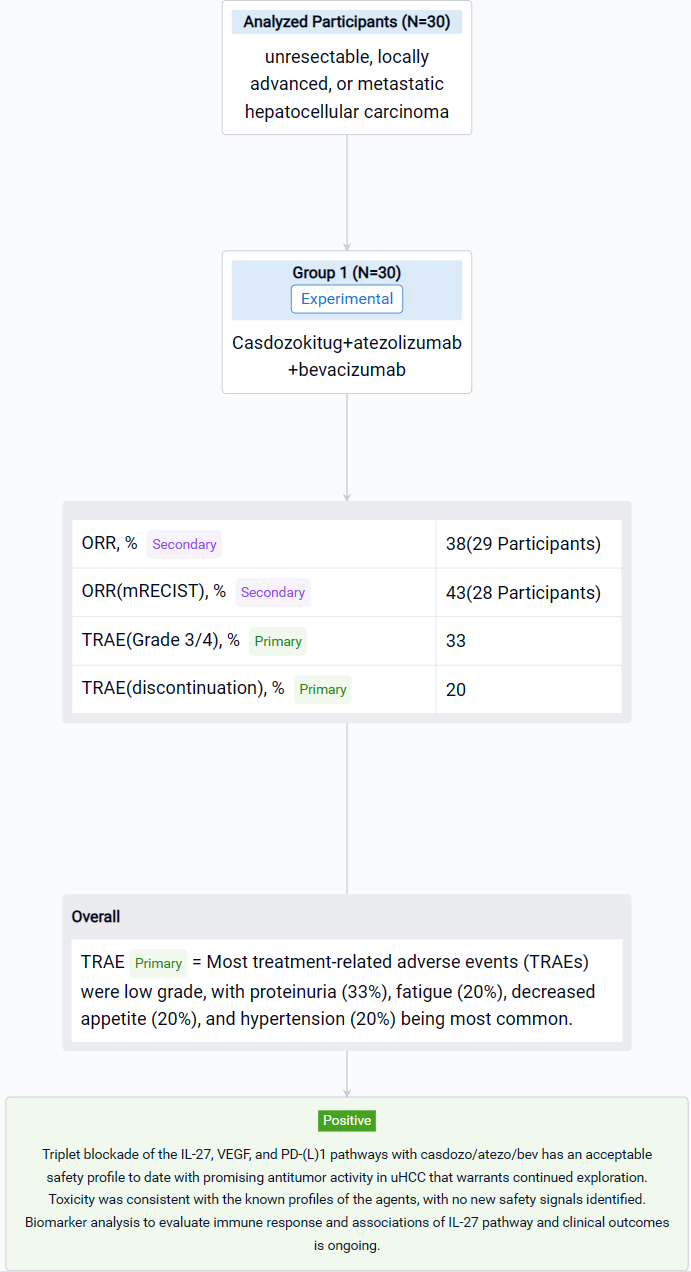
The result showed that as of August 9, 2023, 30 pts were treated with casdozo/atezo/bev. Most pts were male (77%), Asian (67%), ECOG PS 1 (77%), had viral etiology (53% HBV, 17% HCV), and had poor-risk disease as evidenced by metastatic spread (70%) and macrovascular invasion (23%). 47% had AFP ≥400ng/mL. Median time on therapy was 28 wks (3-69). Most treatment-related adverse events (TRAEs) were low grade, with proteinuria (33%), fatigue (20%), decreased appetite (20%), and hypertension (20%) being most common. Grade 3/4 TRAEs occurred in 33%, with only hypertension (13%) arising in ≥10% of pts. TRAEs resulting in any study drug discontinuation was 20%. No deaths were attributed to study drugs. In the 29 response-evaluable pts, 67% experienced any tumor shrinkage with a 38% ORR (3 CRs, 5 confirmed PRs, 3 unconfirmed PRs). In the 28 pts evaluable for mRECIST, ORR was 43% (n=12). More than 50% of pts remain on therapy. Available archival tissue of responders (n=4) all expressed IL-27+ TAMs by IHC, including tumors of viral (n=3) and nonviral etiologies (n=1).
It can be concluded that Triplet blockade of the IL-27, VEGF, and PD-(L)1 pathways with casdozo/atezo/bev has an acceptable safety profile to date with promising antitumor activity in uHCC that warrants continued exploration. Toxicity was consistent with the known profiles of the agents, with no new safety signals identified. Biomarker analysis to evaluate immune response and associations of IL-27 pathway and clinical outcomes is ongoing. Additional clinical studies of casdozo in combination with the PD-1 inhibitor toripalimab are planned.
How to Easily View the Clinical Results Using Synapse Database?
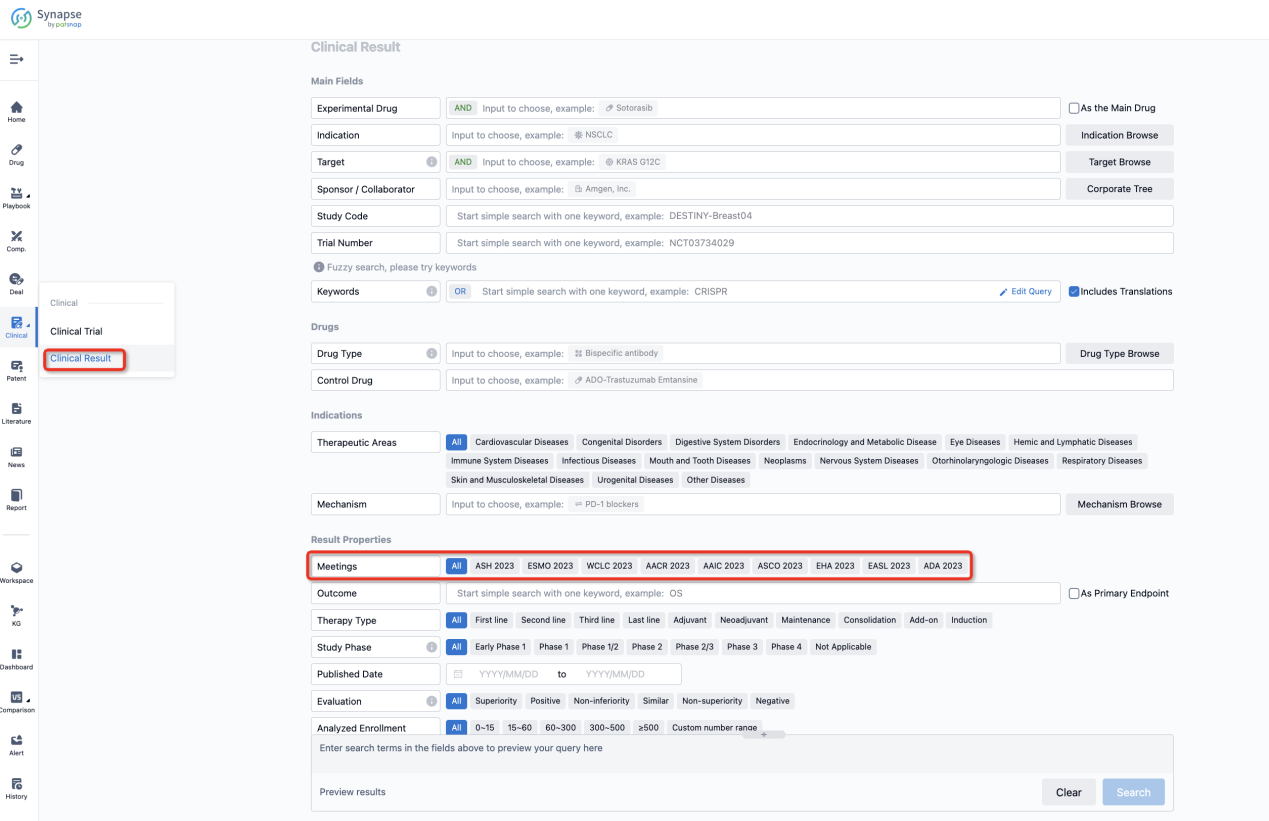
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
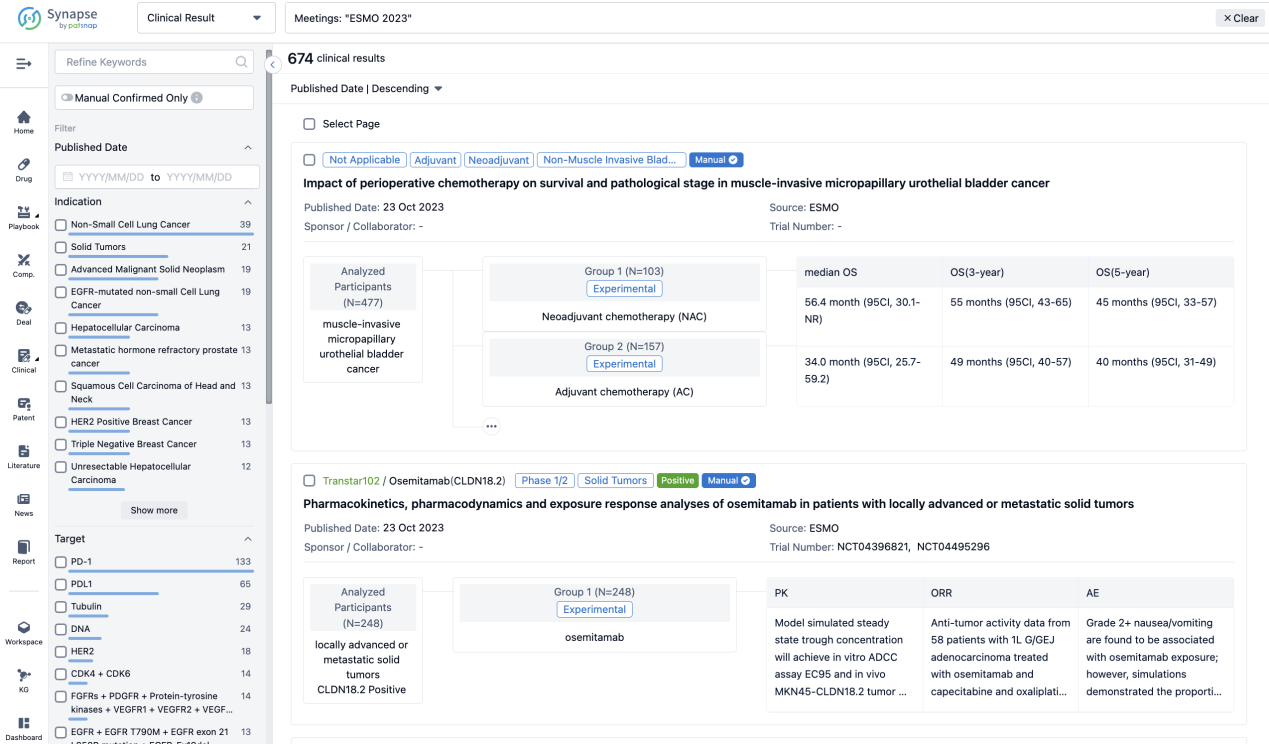
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
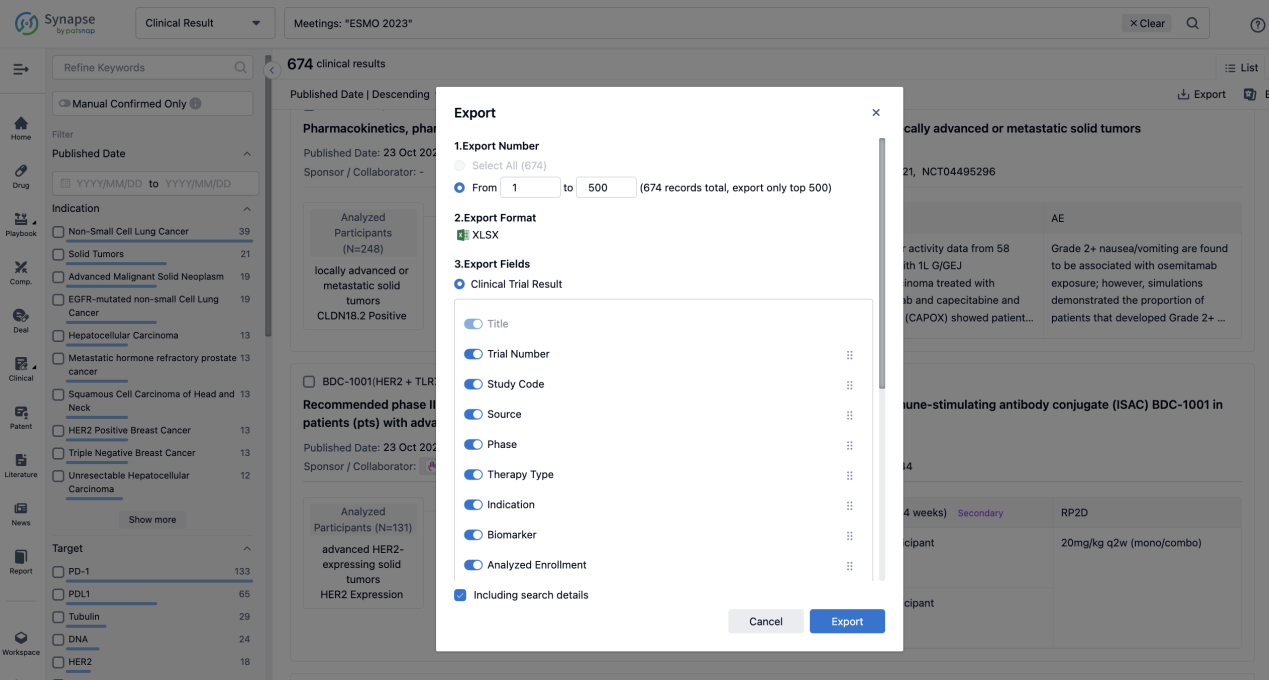
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!