CatalYm's Phase 2a trial for Visugromab in late-stage NSCLC and bladder cancer shows promising results at 2023 ESMO Immuno-Oncology Meeting
CatalYm has made an announcement regarding the progression of their Phase 2a study with the release of advancing results from the GDFATHER-2 trial, which is currently in progress. The trial, whose full title stands for GDF-15 Antibody-mediaTed Human Effector Cell Relocation Phase 2, had key findings shared during a spoken presentation that took place at the 2023 ESMO Immuno-Oncology Congress hosted in Geneva, Switzerland.
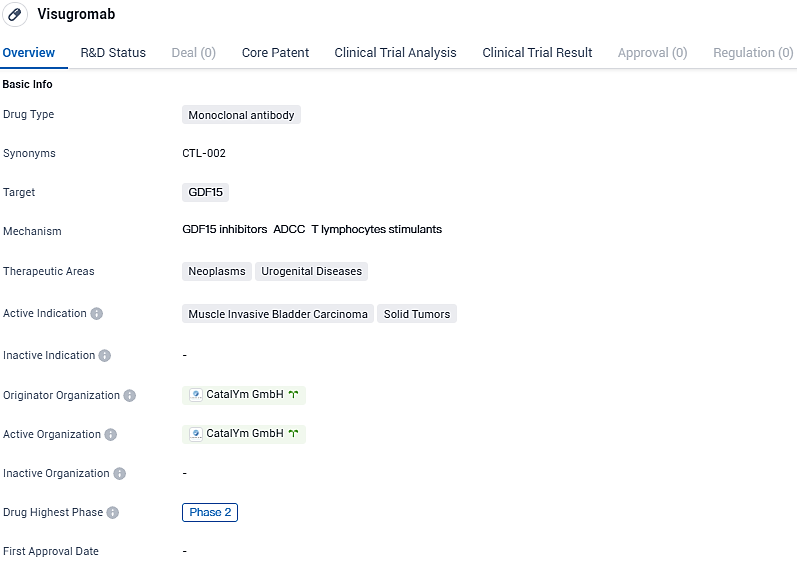
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Research indicates that the pairing of CatalYm's primary investigative drug visugromab with nivolumab presents robust anti-cancer effects in individuals diagnosed with non-small cell lung cancer (NSCLC) and urothelial cancer that have previously shown resistance to treatments targeting PD-1/PD-L1. This therapeutic combination also maintains a favorable safety and tolerability profile. Visugromab is an engineered monoclonal antibody targeting Growth Differentiation Factor-15 (GDF-15), which plays a pivotal role in immunological evasion mechanisms in cancer treatment.
During his speech, Prof. Ignacio Melero, MD, PhD, Co-Director of Immunology and Immunotherapy at Universidad de Navarra in Pamplona, Spain, expanded upon the breadth of visugromab's clinical data. Prof. Melero showcased the potential advantages of inhibiting GDF-15 for patients with metastatic neoplasms.
Prof. Eugen Leo, CatalYm's Chief Medical Officer, asserts that the observed effectiveness and the sustained response especially stand out for advanced and last-line NSCLC and urothelial carcinoma cases that did not respond to prior anti-PD-1/PD-L1 strategies and are undergoing immune-oncology combination therapy.
He notes that the prolonged response—which is still ongoing in 6 out of 8 patients—underscores the potential of this regimen to re-establish enduring immune-mediated control of the tumor in patients with advanced cancer types who have exhausted other conventional treatment options and have become resistant to earlier anti-PD-1/PD-L1 therapies based on stringent criteria. Prof. Leo further noted the significance of these durable responses.
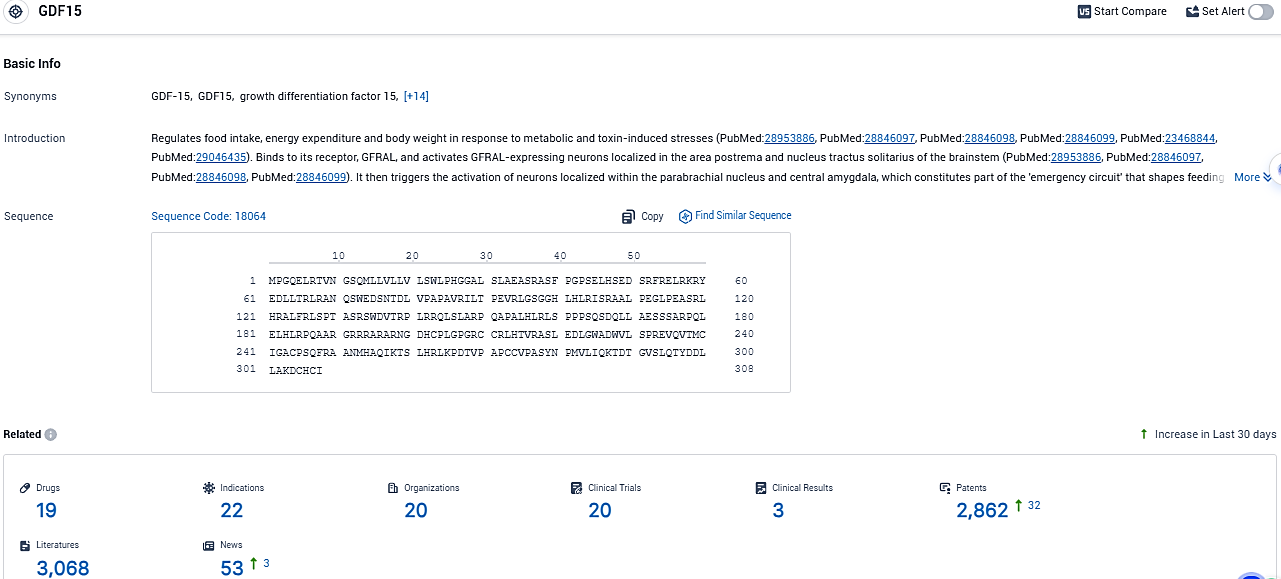
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 13, 2023, there are 19 investigational drugs for the GDF15 target, including 22 indications, 20 R&D institutions involved, with related clinical trials reaching 20, and as many as 2862 patents.
Visugromab is a monoclonal antibody that neutralizes the tumor-derived Growth Differentiation Factor-15. Visugromab has demonstrated already in Phase 1 a good safety profile and potent and durable anti-tumor efficacy in combination with anti-PD-1 treatment in advanced cancer patients. The antibody is currently being investigated in ongoing Phase 2 studies in multiple solid tumor indications.