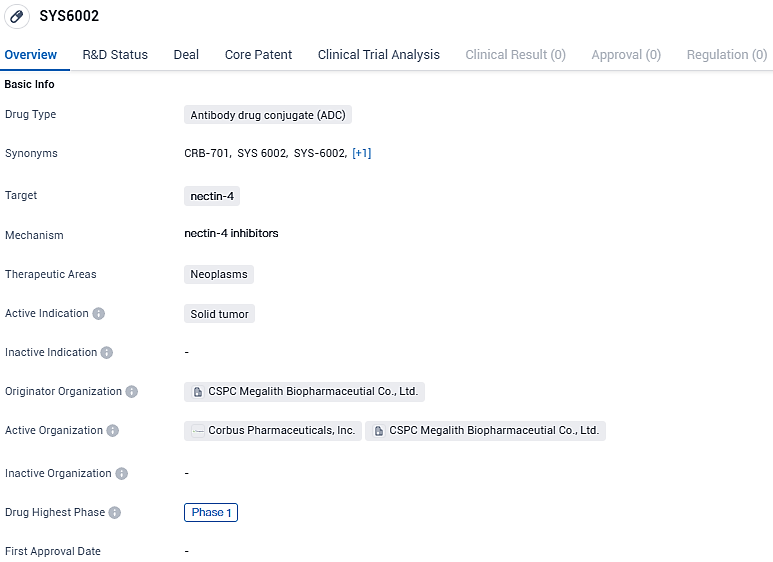
CBR-701 (SYS6002): A Novel Nectin-4-Targeted Therapy Showing Promise in Initial Human Trials for Cancer Patients
Corbus Pharmaceuticals Holdings, Inc. has disclosed that its collaborative partner, CSPC Pharmaceutical Group, is showcasing a poster presentation of the initial human trial findings of CRB-701 (SYS6002) at the 2024 ASCO Genitourinary Cancers Symposium.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
An ongoing Phase 1 clinical trial with a dose expansion phase is actively recruiting patients in China. This trial focuses on individuals diagnosed with metastatic urothelial carcinoma and other solid malignancies confirmed to express nectin-4. Initiated in January of 2023, the trial intends to report findings from the initial set of 18 individuals, representing six varying dosage levels ranging from 0.2 to 3.6 mg/kg, with data collection continuing until December 2023.
Corbus Pharmaceuticals' CEO, Dr. Yuval Cohen, remarked, “Our investigational drug CRB-701 stands out with its innovative monoclonal antibody and advanced linker design, presenting a pharmacokinetic signature distinct from Enfortumab vedotin (EV). Notably, the safety data thus far indicates an absence of serious side effects such as neuropathy and dermal reactions, which are known dose-restrictive toxicities associated with EV. If this trend persists, it could be advantageous for patients with metastatic urothelial carcinoma and for those with other nectin-4 expressing solid tumors, including but not limited to cervical carcinoma.”
Feedback from a leading authority in genitourinary (GU) oncology, Dr. Daniel P. Petrylak, a professor at the Yale School of Medicine with specializations in Medicine and Urology, further supported the therapeutic promise of CRB-701. "The treatment response observed in individuals with nectin-4 positive metastatic urothelial carcinoma and cervical malignancies is promising. Furthermore, the preliminary safety profile is an indication of the drug's potential efficacy in treating various nectin-4 expressing cancers," said Dr. Petrylak.
In his closing statement, Dr. Cohen expressed anticipation for the forthcoming trials scheduled in the United States starting in the first quarter of 2024, thanking CSPC, Corbus's Chinese partner, for their diligent work in executing the current study. He extended appreciation to the medical professionals and participants who are essential to the success of their clinical research.
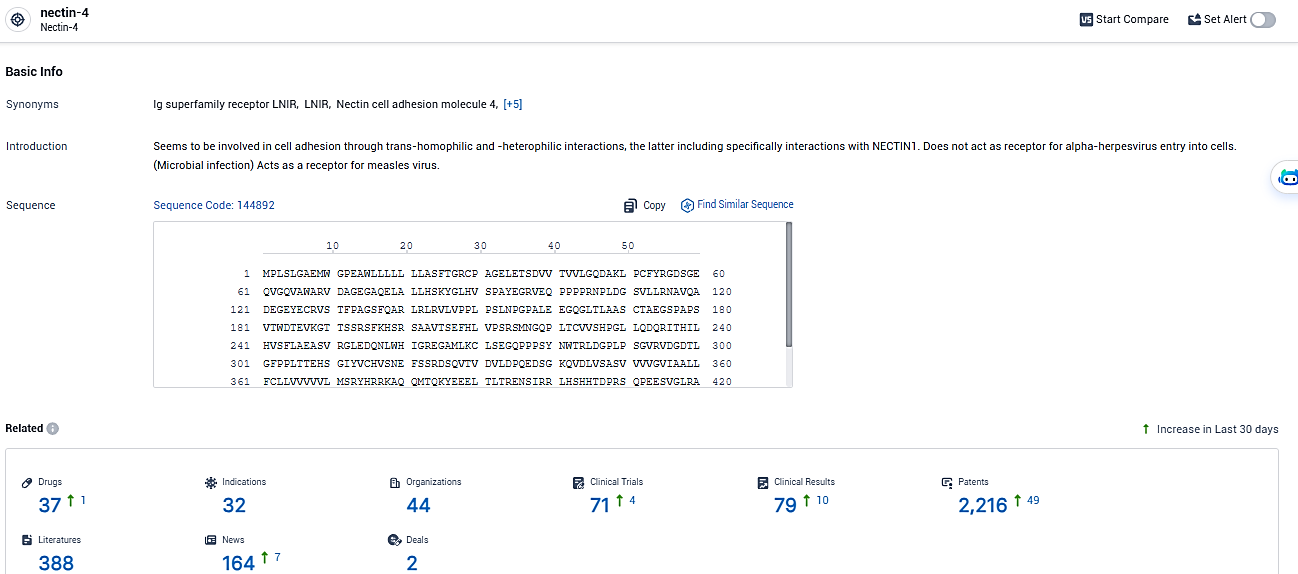
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 30, 2024, there are 37 investigational drugs for the nectin-4 target, including 32 indications, 44 R&D institutions involved, with related clinical trials reaching 71, and as many as 2216 patents.
SYS6002 holds promise in addressing the unmet medical needs associated with solid tumors. However, as it is still in Phase 1 of clinical development, further research and trials are needed to determine its safety and efficacy before it can progress to later stages of development and potentially reach the market.