Flare Therapeutics Reveals FX-909's Early-Stage Trial Design at 2024 ASCO Urologic Cancer Conference
Flare Therapeutics Inc., a pioneer in biotech focusing on developing targeted therapies for oncology and various other conditions by manipulating transcription factors, has revealed their plans to unveil a scientific poster that delineates the design for the initial stage clinical trial of their compound FX-909. This compound stands out due to its remarkable specificity and exceptional efficacy in inhibiting PPARG. The reveal is scheduled during the ASCO Genitourinary Cancers Symposium, an event that will span from January 25th to 27th, 2024, and took place in San Francisco, California.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Flare Therapeutics has developed an extensive array of preclinical findings that justifies progressing FX-909 to human trials," commented Dr. Michael L. Meyers, the company's Chief Medical Officer. "Our preliminary outcomes indicate that FX-909 possesses potent antitumor efficacy against urothelial carcinoma in murine models with minimal oral dosages. Our research team has further exploited increased expression of PPARG in defining a subtype of muscle-invasive bladder cancer (UC) and in the pursuit of specific genetic patient groups."
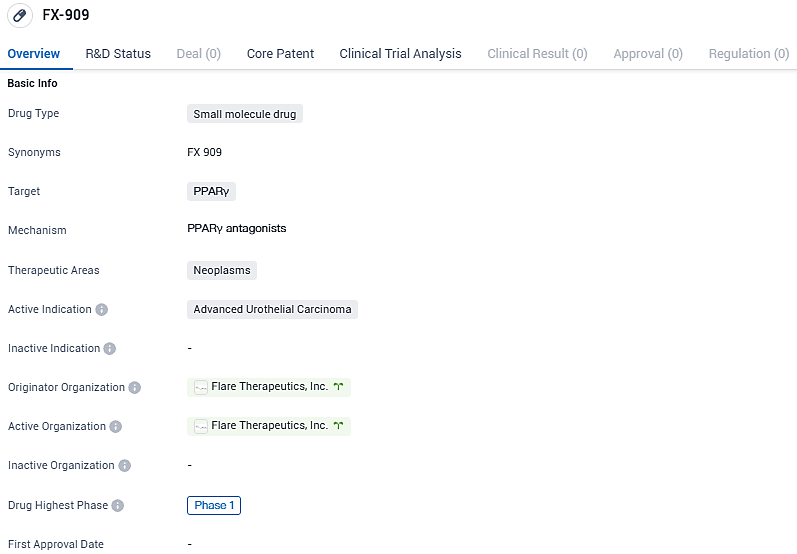
PPARG is a critical determinant of the luminal phenotype and is implicated in the majority—approximately two-thirds—of all advanced UC cases. The strategic disruption of PPARG signaling represents a cutting-edge tactic in managing advanced UC, potentially setting the stage for better patient prognoses, particularly in those with luminal disease. The therapeutic application of FX-909 to distinct UC xenograft models demonstrated a substantial 84% hindrance in tumor proliferation, correlating to an anticipated human equivalent dose of 50 mg – the initial dose in early human studies (Phase 1).
Dr. Gopa Iyer, a Genitourinary Oncologist and specialist in novel drug development at Memorial Sloane Kettering Cancer Center, noted, "Despite the high mortality associated with advanced bladder cancer and muscle-invasive UC, recent strides in understanding the disease's molecular intricacies have ushered in a resurgence of innovative therapeutic alternatives."
"Furnished with FlareTx's unique mode of action, there's the potential for a desperately needed alternative treatment for patients who experience disease progression post initial treatments, which may include platinum-based chemotherapy, immune checkpoint inhibitors, and ADC-directed treatments," Iyer continued.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
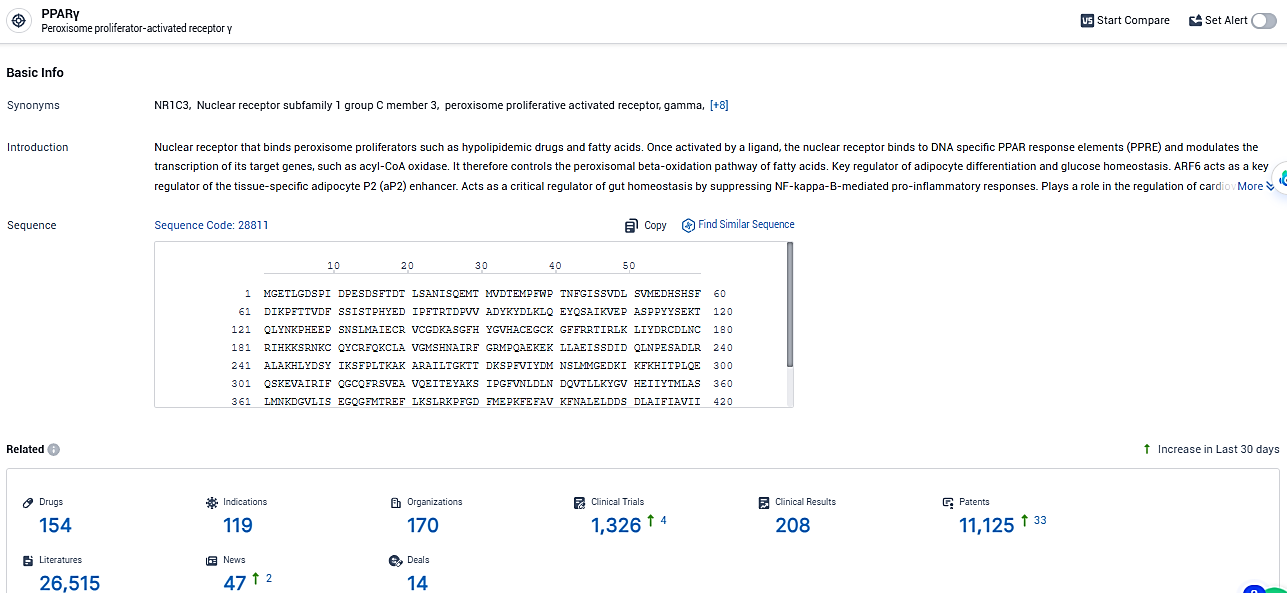
According to the data provided by the Synapse Database, As of January 30, 2024, there are 154 investigational drugs for the PPARγ target, including 119 indications, 170 R&D institutions involved, with related clinical trials reaching 1326, and as many as 11125 patents.
FX-909, is a first-in-class, highly potent and selective small molecule that inhibits the transcription factor peroxisome proliferator-activated receptor gamma (PPARG) to treat patients with the luminal subtype of advanced urothelial carcinoma and potentially other solid tumors. Further research and clinical trials will be required to determine the drug's efficacy and potential as a treatment option for patients with this specific type of cancer.