Celltrion Shows Promising Phase III Results for RA Biosimilar CT-P47 at EULAR 2024
At the 2024 Annual European Congress of Rheumatology, Celltrion unveiled encouraging results from a Phase III study of CT-P47, a biosimilar developed in comparison to RoActemra, targeting individuals with moderate-to-severe rheumatoid arthritis. The findings from the Phase III comparative clinical trial highlighted that CT-P47 offers similar efficacy and exhibits a comparable safety and immunogenicity profile to the reference tocilizumab (RoActemra) product.
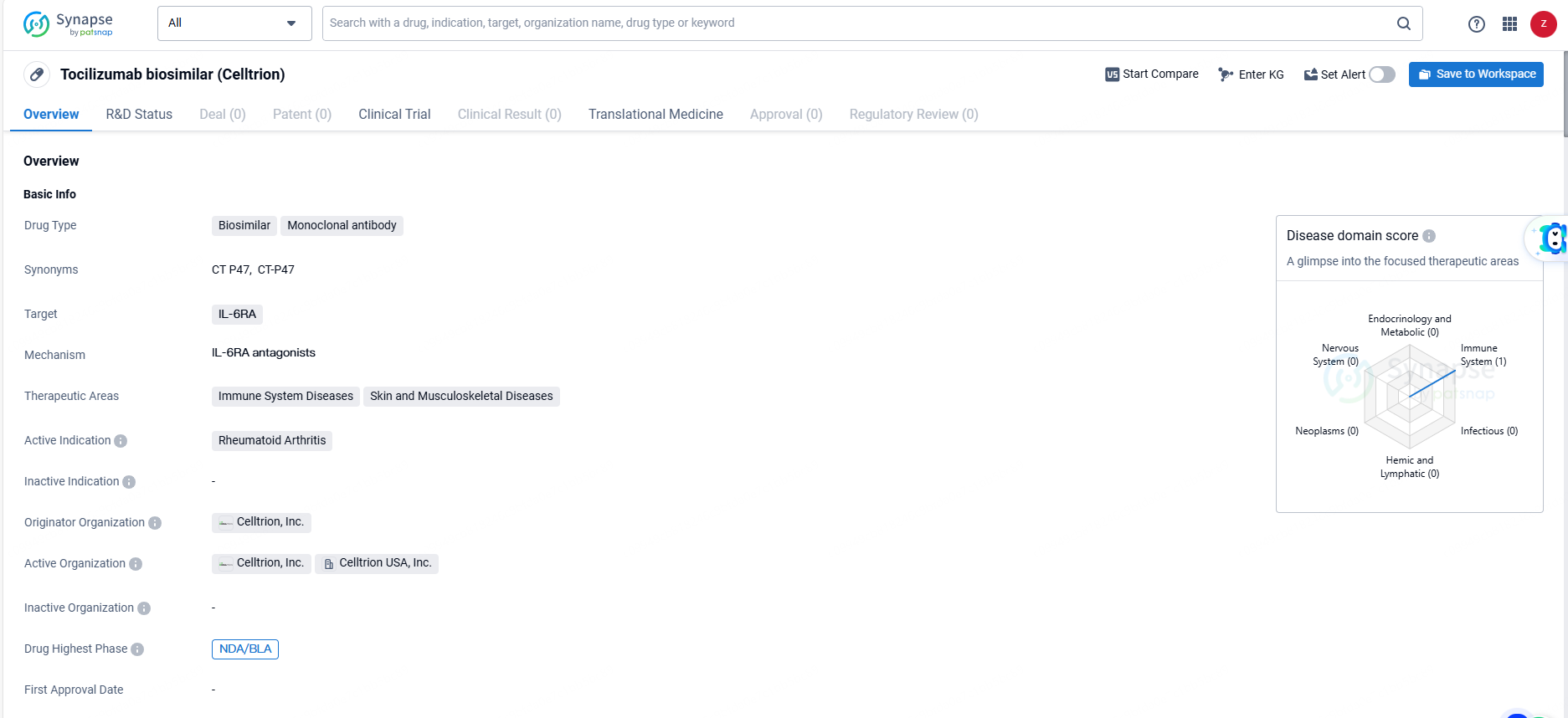
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
In the Phase III trial, 471 patients with moderate to severe rheumatoid arthritis (RA) were randomly assigned to receive either CT-P47 or reference tocilizumab every four weeks at a dosage of 8 mg/kg for the initial 20 weeks. Before the 24th week of treatment, those on tocilizumab were re-randomized to either continue with the reference tocilizumab or switch to CT-P47, continuing up to week 48.
The primary endpoint was the improvement in the Disease Activity Score 28 erythrocyte sedimentation rate at weeks 12 and 24. The calculated differences between the two treatment groups were -0.01 at week 12 and -0.1 at week 24. The confidence intervals for these differences were fully contained within the pre-defined equivalence margins at both time points.
Both treatment groups displayed remarkable similarity in terms of average serum concentration up to week 32, frequency of treatment-emergent adverse events, and anti-drug antibody development, confirming comparable pharmacokinetics, safety, and immunogenicity.
"Biosimilars offer a significant opportunity to meet unmet medical needs by broadening access to high-quality biologic medications. The positive top-line outcomes from this Phase III study affirm the biosimilarity of CT-P47 to reference tocilizumab and also provide clinical evidence supporting the possibility of transitioning from reference tocilizumab to CT-P47," stated Dr. Josef S. Smolen, Emeritus Professor of Medicine at the Medical University of Vienna in Austria.
CT-P47, which includes the active ingredient tocilizumab, is a recombinant humanized monoclonal antibody functioning as an interleukin 6 (IL-6) receptor antagonist. Based on results from the global Phase III trial, which was designed to assess the efficacy, pharmacokinetics, safety, and immunogenicity of CT-P47 in comparison to the reference product RoActemra, CT-P47 was submitted for regulatory approval with the U.S. Food and Drug Administration and the European Medicines Agency in January and February 2024, respectively.
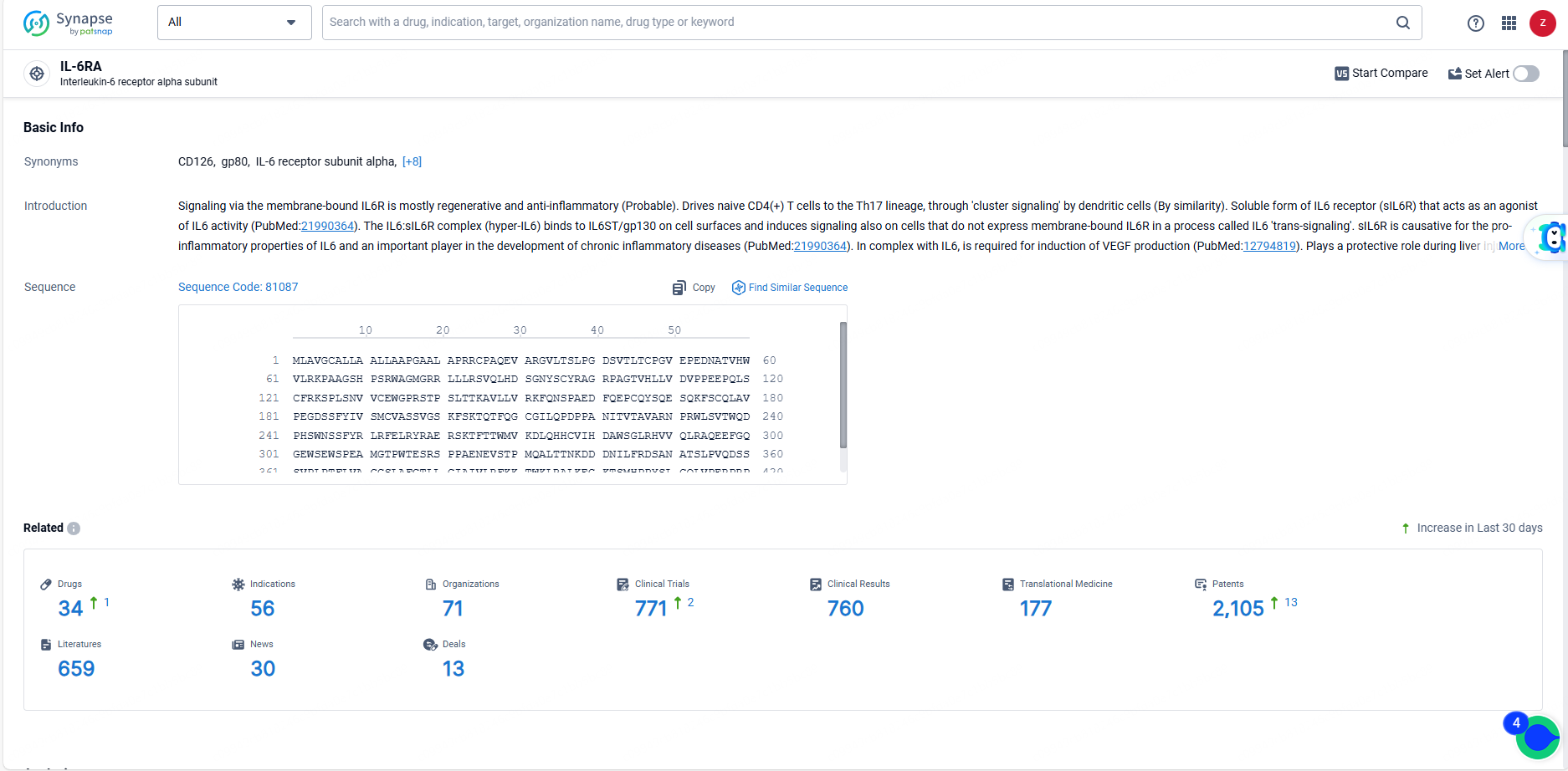
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 18, 2024, there are 34 investigational drugs for the IL-6RA target, including 56 indications, 71 R&D institutions involved, with related clinical trials reaching 771, and as many as 2105 patents.
Tocilizumab is a promising option for the treatment of rheumatoid arthritis, a chronic autoimmune condition that affects the joints and can lead to significant disability if not properly managed. By inhibiting the action of IL-6RA, the drug aims to modulate the immune response and reduce inflammation, thereby alleviating the symptoms and slowing the progression of the disease.